For each of the following unbalanced equations, indicate how many moles of the second reactant would be required to react exactly with 0.275 mole of the first reactant. Balance the equation firs
For each of the following unbalanced equations, indicate how many moles of the second reactant would be required to react exactly with 0.275 mole of the first reactant. Balance the equation firs
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter9: Nuclear Chemistry
Section: Chapter Questions
Problem 9.10P: 9-10 Microwaves are a form of electromagnetic radiation that is used for the rapid heating of foods....
Related questions
Question
For each of the following unbalanced equations, indicate how many moles of the second reactant would be required to react exactly with 0.275 mole of the first reactant. Balance the equation first!
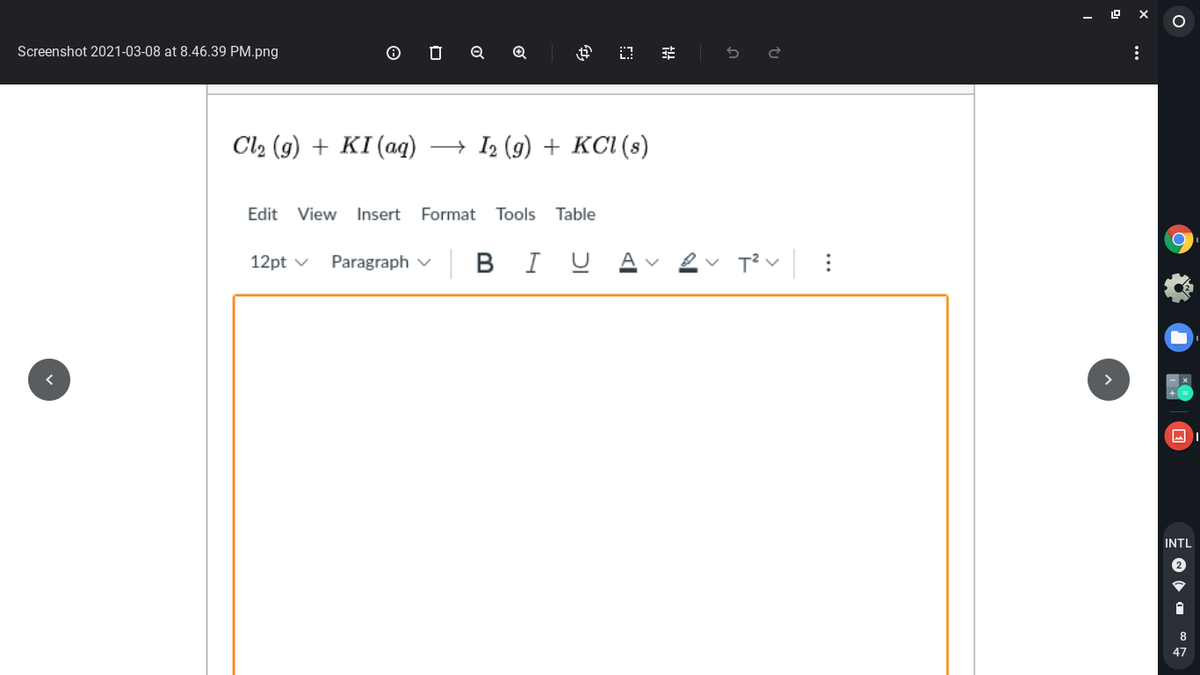
Transcribed Image Text:Screenshot 2021-03-08 at 8.46.39 PM.png
Cla (9) + KI (аg)
+ I2 (g) + KCI (s)
Edit View
Insert Format Tools
Table
Paragraph v
|BIU
12pt v
INTL
47
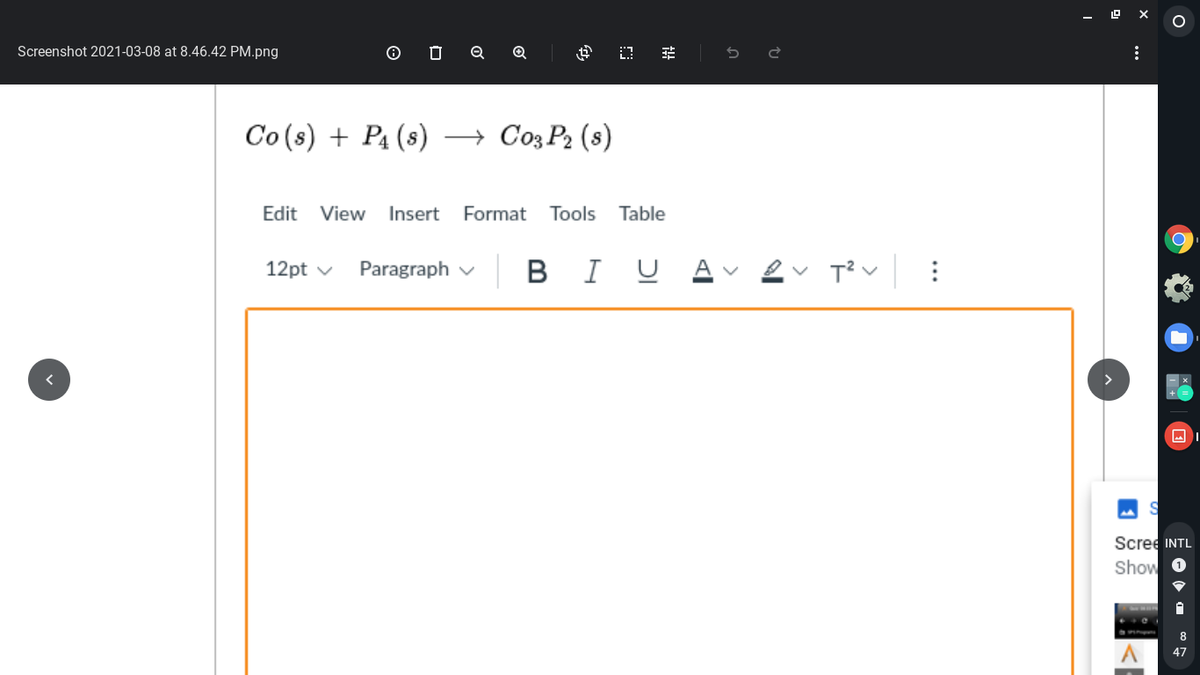
Transcribed Image Text:Screenshot 2021-03-08 at 8.46.42 PM.png
Co (s) + P4 (s)
→ Co3 P2 (s)
Edit
View
Insert
Format
Tools
Table
B
I U
Av 2v T v:
12pt v
Paragraph v
Scree INTL
Show
8
47
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning