For each quantity in the table below, write the symbol of the SI unit in which it would usually be measured. quantity unit symbol The exposure to radiation experienced by observers of a nuclear weapons test. 0 The levels of radiation detected by a Geiger counter when brought near a 1 g. sample of radium. 0 The total dose of gamma radiation typically used to treat prostate cancers of a certain stage. 0 The intensity of nuclear radiation emitted by a gram of iodine-131. 0
For each quantity in the table below, write the symbol of the SI unit in which it would usually be measured. quantity unit symbol The exposure to radiation experienced by observers of a nuclear weapons test. 0 The levels of radiation detected by a Geiger counter when brought near a 1 g. sample of radium. 0 The total dose of gamma radiation typically used to treat prostate cancers of a certain stage. 0 The intensity of nuclear radiation emitted by a gram of iodine-131. 0
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 16QRT
Related questions
Question
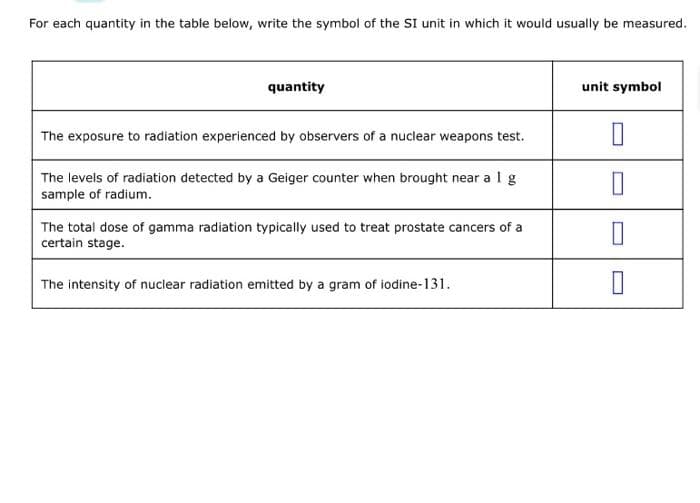
Transcribed Image Text:For each quantity in the table below, write the symbol of the SI unit in which it would usually be measured.
quantity
unit symbol
The exposure to radiation experienced by observers of a nuclear weapons test.
0
The levels of radiation detected by a Geiger counter when brought near a 1 g
sample of radium.
0
The total dose of gamma radiation typically used to treat prostate cancers of a
certain stage.
0
The intensity of nuclear radiation emitted by a gram of iodine-131.
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning