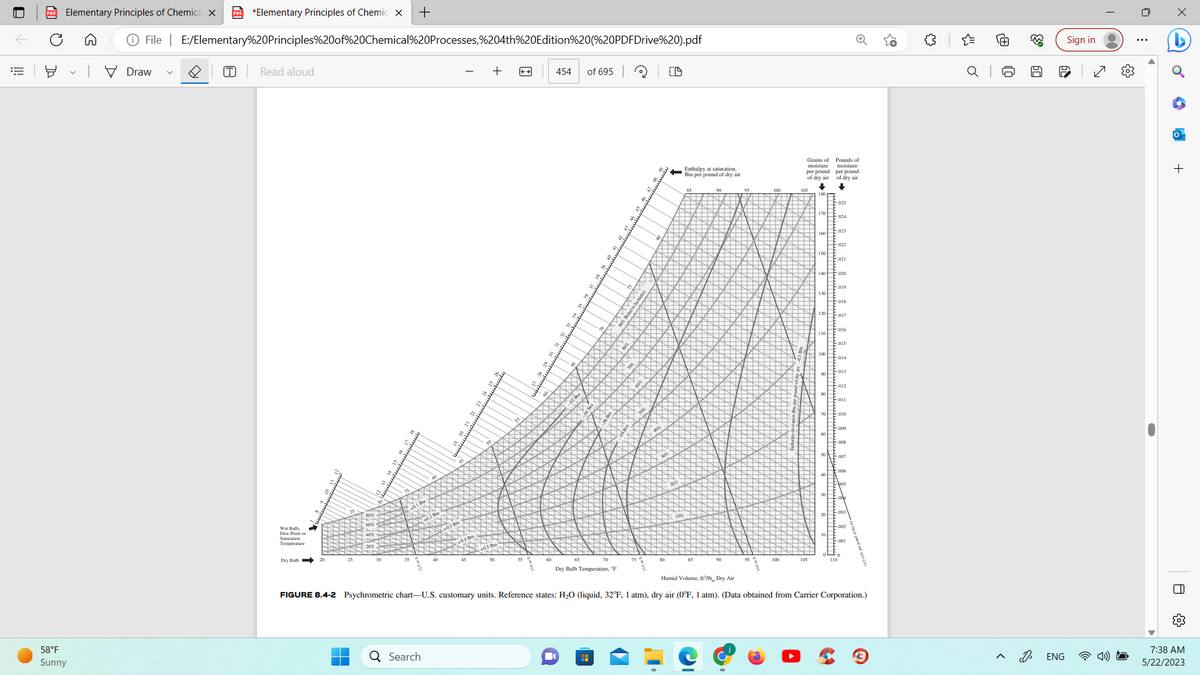
For point 1, I cannot locate the humidity and enthalpy. For point 2, I cannot locate the humidity and enthalpy. Where do you locate these on the graph. The graph is provided for this.
For point 1, I cannot locate the humidity and enthalpy. For point 2, I cannot locate the humidity and enthalpy. Where do you locate these on the graph. The graph is provided for this.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
For point 1, I cannot locate the humidity and enthalpy. For point 2, I cannot locate the humidity and enthalpy. Where do you locate these on the graph. The graph is provided for this.
Transcribed Image Text:||!
PDF Elementary Principles of Chemica X PDF *Elementary Principles of Chemic X +
58°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
Wet Bulb,
Dew Point or
Saturation
Temperature
Dry Bulb 20
25
==
i
Q Search
2
23
=
S
55
24
454 of 695
8
%
60
A
3
65
R
R
#
9:
2
Dry Bulb Temperature, F
1
4
$.
5.
2.
7
CD
Enthalpy at saturation,
of dry air
per
85
Humid Volume, ft/lb Dry Air
95
Grains of Pounds of
moisture
mais
moisture
per pound.
per pound
of dry air
of dry air
105
110
025
120017
90
024
014
70010
006
002
FIGURE 8.4-2 Psychrometric chart-U.S. customary units. Reference states: H₂O (liquid, 32°F, 1 atm), dry air (0°F, 1 atm). (Data obtained from Carrier Corporation.)
{"
Ơ
Ⓡ
63
+
60
D
ENG
Sign in
(0)
+
O
7:38 AM
5/22/2023
Transcribed Image Text:||!
PDF Elementary Principles of Chemica X PDF *Elementary Principles of Chemic X +
58°F
Sunny
File | E:/Elementary%20Principles%20of%20Chemical%20Processes,%204th%20Edition%20(%20PDFDrive%20).pdf
Draw
T Read aloud
8.70
+
458 of 695 (2) OD
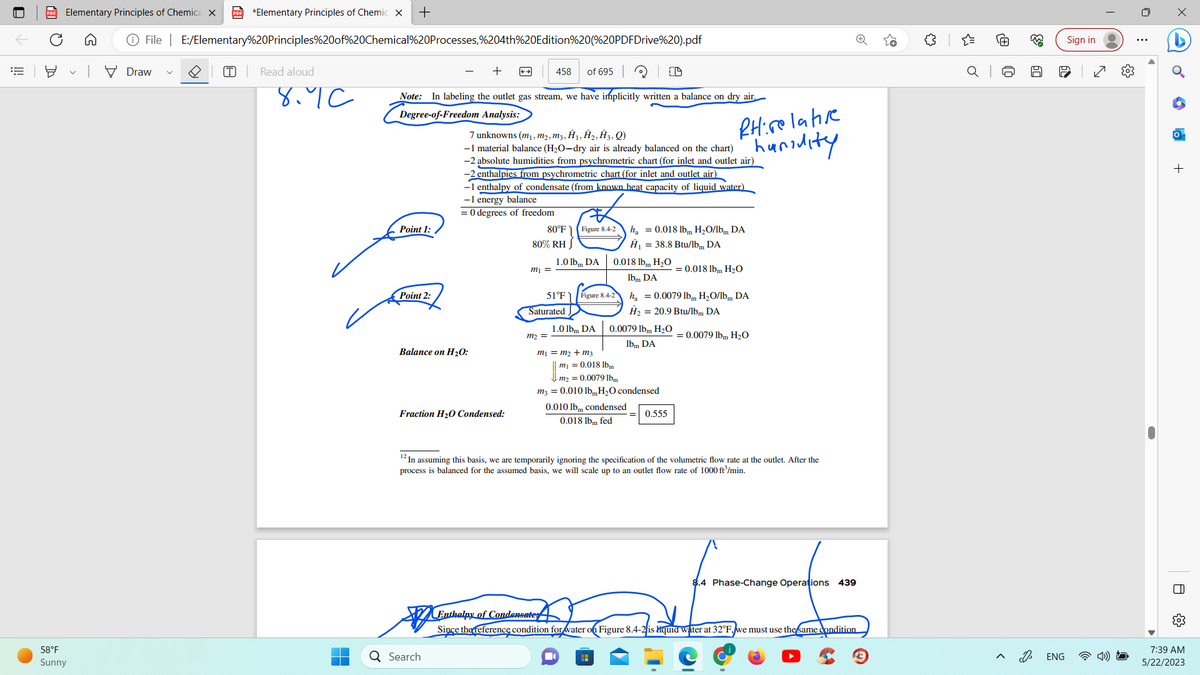
Note: In labeling the outlet gas stream, we have implicitly written a balance on dry air.
Degree-of-Freedom Analysis:
Point 1:
Point 2:
7 unknowns (m₁, m₂, m3, Ĥ₁, Ĥ₂, Ĥ3, Q)
-1 material balance (H₂O-dry air is already balanced on the chart)
-2 absolute humidities from psychrometric chart (for inlet and outlet air)
-2 enthalpies from psychrometric chart (for inlet and outlet air)
Balance on H₂O:
-1 enthalpy of condensate (from known heat capacity of liquid water)
-1 energy balance
= 0 degrees of freedom
Fraction H₂0 Condensed:
Q Search
80°F
80% RH
m₁ =
Figure 8.4-2
1.0 lbm DA
Saturated
m₂ =
51°F Figure 8.4-2
1.0 lbm DA
m₁ = m₂ + m3
0.018 lbm H₂O
lbm DA
ha = 0.018 lb H₂O/lbm DA
Ĥ₁ = 38.8 Btu/lbm DA
0.010 lbm condensed
0.018 lbm fed
RHirelative
haridity
0.0079 lbm H₂O
lbm DA
m₁ = 0.018 lbm
m₂ = 0.0079 lbm
m3 = 0.010 lb, H₂O condensed
H
h₁ = 0.0079 lbm H₂O/lbm DA
Ĥ₂ = 20.9 Btu/lbm DA
0.555
= 0.018 lbm H₂O
12 In assuming this basis, we are temporarily ignoring the specification of the volumetric flow rate at the outlet. After the
process is balanced for the assumed basis, we will scale up to an outlet flow rate of 1000 ft³/min.
= 0.0079 lbm H₂O
pa
Enthalpy of Condensate
Since the reference condition for water on Figure 8.4-2 is liquid water at 32°F, we must use the same condition
8.4 Phase-Change Operations 439
{"
Ơ
Ⓡ
J
63
60
D
ENG
Sign in
(0)
+
O
7:39 AM
5/22/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The