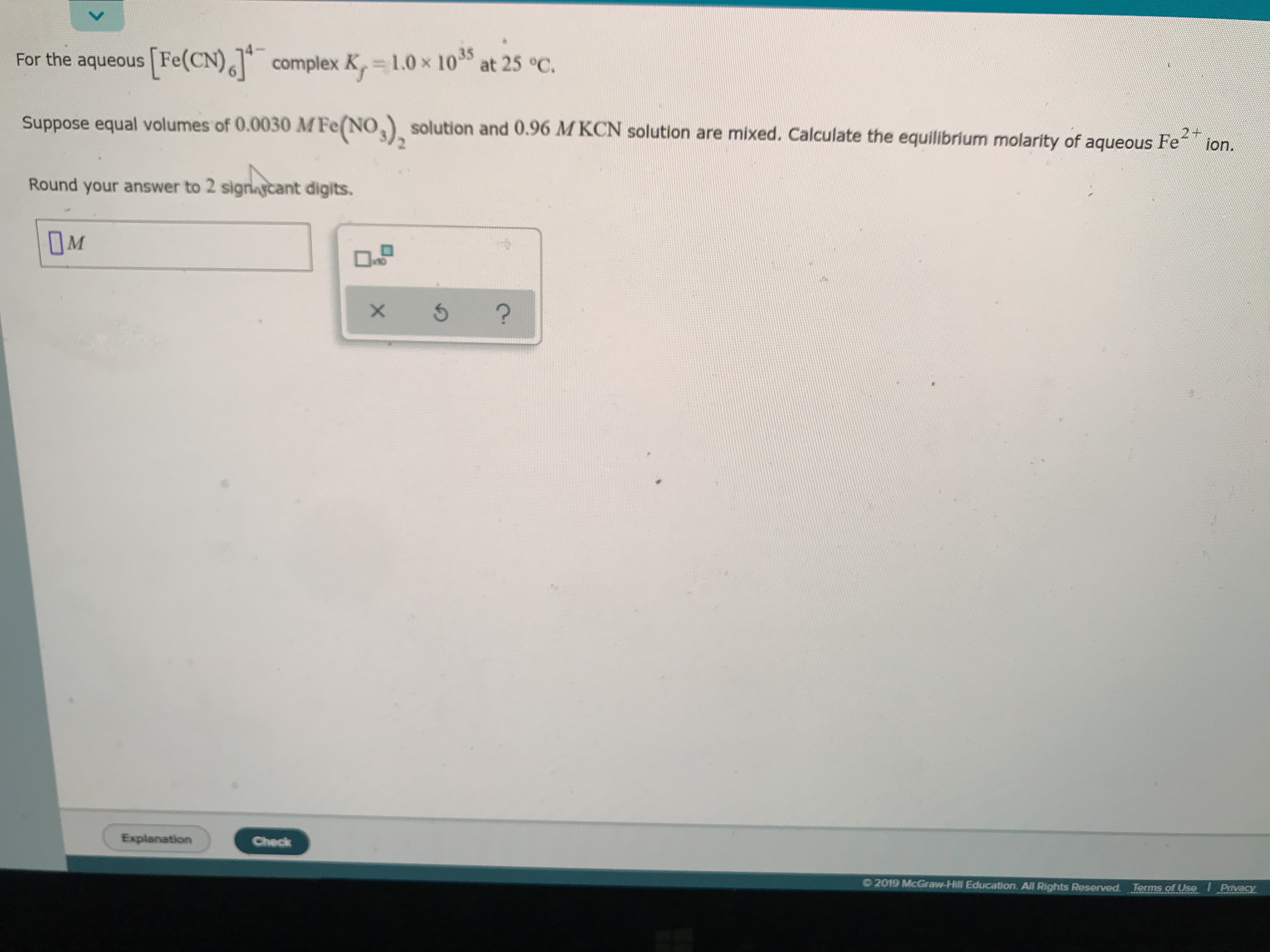
For the aqueous Fe(CN)complex K, 1.0x 1035 at 25 °C. Suppose equal volumes of 0.0030 MFe(NO solution and 0.96 MKCN solution are mixed. Calculate the equilibrium molarity of aqueous Fe ion. Round your answer to 2 signaycant digits. M ? Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Pivacy X
Q: Agl is a slightly soluble salt with Ksp = 1.5x1016, In presence of NH3 silver ion forms the complex…
A: Given, Solubility product constant (Ksp) of AgI = 1.5 x 10-16 Formation constant of Ag(NH3)2+ = Kf…
Q: Calculate the solubility at 25 °C of PbCo, in pure water and in a 0.0050 M Pb(NO,) solution. You'l…
A:
Q: Consider the reversible formation of the C u ( N H 3 ) 4 2 + complex ion. C u 2 + ( a q ) + 4 N H 3…
A:
Q: Calculate the solubility at 25 "C of PhCO, in pure water and in a 0.0020 M Pb(NO,), solution. You'l…
A:
Q: For the aqueous Fe(CN), complex K, = 1.0 x 10" at 25 "C. Suppose equal volumes of 0.0046M Fe(NO,),…
A: The equilibrium constant for a reaction is expressed in terms of the ratio of the concentration of…
Q: The solubility in mol/L of Ag2CrO4 is 1.0 × 10–4 M at a certain temperature (i.e., what is…
A: Given solubility of Ag2CrO4 = 1.0 × 10-4 M
Q: The Ksp of Fe3(PO4)2 is 2.22⋅10^−25 M. Calculate the solubility of each ion in a saturated…
A: Ksp is the solubility product constant of any solid dissolving in aqueous solution. It represents…
Q: Calculate the solubility of a sparingly soluble salt AX2 in a solution containing 0.065 M NaX.…
A:
Q: Calculate the solubility of Ba(IO3)2 in pure water, Ksp=1.5x10^-9. Then calculate the solubility of…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Calculate the solubility at 25 °C of PbCO, in pure water and in a 0.0080M Pb(NO,), solution. You'll…
A: At 25℃, Ksp of PbCO3=7.40×10-14 [Pb(NO3)2] =0.0080 M Molar mass of PbCO3=267.21 g mol-1
Q: Calculate the solubility at 25 °C of CaF, in pure water and in a 0.0130M NaF solution. You'll find K…
A:
Q: Calculate the solubility at 25 °C of Co(OH), in pure water and in a 0.0180M CoCl, solution. You'll…
A:
Q: 5.2 Calculate the solubility of CaF2 (g/L) in a solution of KF (0.15 M). Show full reasoning
A: Solubility product :- It is the equilibrium constant whose value get increases with increase in…
Q: Calculate the solublity at 25 °C of CaF, In pure water and In a 0.0120M NaF solution. You'll find K…
A:
Q: Given the equation Ag*(aq) + 2 NH,(aq) [Ag(NH,),]*(aq) Kf = 2.00 × 107 determine the concentration…
A: Given: Mass of AgCl to be dissolved = 529 mg = 0.529 g. Volume of solution = 100.0 mL = 0.100 L…
Q: How many moles of PbCl2 (Ksp = 1.6 x 10) will dissolve in 50.0 ml of .10M AICI3? (hint: solve for…
A: Given, Solubility product (Ksp) of PbCl2 = 1.6 x 10-5 Volume of AlCl3 = 0.10 M Number of moles of…
Q: Look at sample problem 19.14 in the 8th ed Silberberg book. In black-and-white film developing,…
A: AgBr (s) ⇌ Ag+ + Br-Ag+ + 2S2O32-(aq) ⇌ [Ag(S2O3)2] The overall reaction isAgBr + 2S2O32-…
Q: F7. Cobalt (II) carbonate is allowed to dissolve to saturation in an aqueous solution also…
A: Solution - According to the question - Given - Given equation - Co + 6NH3 = [Co(NH3)]2+ + Co3. Kf =…
Q: What is the molar solubility of AgCI (Ksp = 1.80 × 10-1º) in 0.600 M NH,? (Kf of Ag(NH,),* is 1.7 ×…
A: MOLAR SOLUBILITY Molar solubility (mol/L)is defined as the number of moles of solute dissolved in 1…
Q: Calculate the solubility at 25 °C of AgCl in pure water and in a 0.0100 M AGNO, solution. You'll…
A:
Q: For the aqueous AIF complex K= 6.92 x 10" at 25 °C. .3+ Suppose equal volumes of 0.0076M Al (NO,),…
A:
Q: Determine the molar solubility of copper(I) azide (CuN, ) in a solution with a pH of 2.333. Ignore…
A:
Q: For the aqueous Hg(NH,), complex K, =1.8 x 10" at 25 °C. !! Suppose equal volumes of 0.0042 M…
A: Since equal volume is added, therefore molarity will become half of initial value. Hg2+ +…
Q: Hg2I2(s)⇄Hg22+(aq)+2I−(aq) Ksp=[Hg22+][I−]2 A saturated solution of Hg2I2 is at equilibrium at 25°C…
A: Here [Hg22+] produced in the reaction = 1/2 [I-] = (4.6 x 10-10) / 2 = 2.3 x 10-10 M
Q: 19 For the aqueous , complex K,=4.0 × 10" at 25 °C. AlF Suppose equal volumes of 0.0050M Al(NO,),…
A:
Q: calculate the ksp for PbBr2 if it's molar solubility is 2.14x10^-2 M at 25 degrees celsius. show…
A:
Q: Number 2
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and…
Q: calculate the sulablity Iat 25 Celsius of Ni(OH)2 pure water and then .01 80 M NaOH solution. Ksp…
A: Given:Concentration of NaOH = 0.0180M.Ksp of Ni(OH)2 =5.48×10−16
Q: (250ml. Flash ined with 0.50 mot of Hn and 1.2mol of HCI. -M₂g) + Cl₂(g) = 2 Hd (₂) equilibrium…
A:
Q: 7.00 mL of 8.00 x 103 M Fe(NO3)3 (8.00 times 10 to the minus 3rd power M Fe (NO3)3) is added to 6.00…
A: Total concentration of [Fe(SCN)2+] at equilibrium = 1.00×10-4 Thus the concentration of Fe3+ at…
Q: Calculate the solubility at 25 °C of Zn (OH), in pure water and in a 0.0100M ZnSO, solution. You'lIl…
A: From alesk data, Ksp of Zn(OH)2=3.0×10-17 Molar mass of Zn(OH)2=99.424 g mol-1
Q: ne solubility of Ce(IO3)3 in a 0.26-M KIO3 solution is 2.0 x alculate Kp for Ce(IO3)3 - sp
A:
Q: Does the NaOH raise or lower the solution pH? What is the amount of NaOH added in lbs/ton? What is…
A: Volume of solution = 200 gallons 1 gallon = 0.0315 ton So, Volume = 6.3 ton We have added NaOH to…
Q: Calculate the solubility at 25 °C of CaF, in pure water and in a 0.0110M NaF solution. You'll find…
A:
Q: Calculate the solubility at 25 °C of CaF, in pure water and in a 0.0110M NaF solution. You'll find…
A: Given, Concentration of NaF, [NaF] = 0.0110 M Ksp of CaF2 = 3.45x10-11 Molar mass of CaF2 = 78.07…
Q: The Ksp value of the ionic comnpound MX3 is 2.5 × 10-18 at 25 °C. MX3(s) = M³+(aq) + 3X (aq) a.…
A: a) Since the Ksp expression for the solubility reaction of MX3 can be written as => Ksp = [M3+]…
Q: If a solid AB2 is added to water, and at equilibrium the concentration of A2+ is 6.23 x 10, what is…
A: Solubility product is the parameter to understand the amount of solubility of a salt.
Q: The Ksp value of the ionic comnpound MX3 is 2.5 × 10-18 at 25 °C. MX3(s) = M³+(aq) + 3X (aq) a.…
A: Given : Ksp value for MX3 = 2.5 × 10-18 Concentration of NaX = 0.15 M
Q: The Ksp for Ag,SO4 is 1.4 x 105 at 25.0°C Write the dissociation equation for Ag2SO4. а. b. Complete…
A: a.) Ag2SO4 is a sparingly soluble salt , so it will not dissociate completely into its ions . Thus…
Q: Calculate the solubility at 25 °C of AgCl in pure water and in a 0.0020M AGNO, solution. You'll find…
A: Ksp of AgCl = 1.8 x 10-10 Concentration of AgNO3 = 0.0020 M Molar mass of AgCl = 143.5 gm/mole
Q: It is found that up to 0.0110 g of SrF₂ dissolves in 100 mL of aqueous solution at a certain…
A: Solubility product (Ksp)- It is equilibrium constant for a chemical reaction in which a solid ionic…
Q: For the aqueous Ni(CN),] complex K,=2.00 × 10" at 25 °C. Suppose equal volumes of 0.0090M Ni(NO,)…
A: Given: Kf for the complex [Ni(CN)4]2- = 2.00×1031 It is given that equal volumes of 0.0090 M…
Q: Thallium(I) iodate (TlIO3) is only slightly soluble in water. Its Ksp at 25°C is 3.07 × 10-6 .…
A: Solubility is defined as the ability of a solute to dissolve in the solvent. Different substances…
Q: 4- For the aqueous Fe(CN), complex K,=1.0 × 10 35 at 25 °C. Suppose equal volumes of 0.0068 M…
A: Equal volume of Fe (NO3)2 and KCN = x L Total volume of solution = (x + x) L…
Q: Calculate the solublity at 25 °C of Ni(OH), In pure water and In a 0.0040M NaOH solution. You'll…
A:
Q: For the aqueous [Hg(NH3)4]²* complex K, = 1.91 × 10¹⁹ at 25 °C. Suppose equal volumes of 0.0062 M…
A:
Q: Calculate the solubility at 25 °C of AgBr in pure water and in 0.39 M ammonia (NH,). You'll probably…
A: Step 1: In first case, considering that the AgCl dissociates in pure water and does not react with…
Q: Consider the insoluble compound silver hydroxide , AgOH. The silver ion also forms a complex with…
A: The solubility product of salt is a constant at a given temperature irrespective of the source from…
Q: Kf for the complex ion Ag(NH3)2+ is 1.5 × 107 at 25 ° C. Using this information, calculate the value…
A: from thermodynamics ∆G° = -RT ln Keq
Calculate the equilibrium molarity of aqueous Fe^2+
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

- 1.1The Ksp of Ca3 (PO4 ) 2 is 1.3 × 10−26 . Estimate the solubility of this salt in units of g. L −1 . You must show any reaction equation(s) that you may think are necessary. 1.2 If a sample of solid Ca3(PO4)2 is stirred into exactly one litre of a 0.550M solution of Na3PO4, how will the solubility of the salt compare with the answer that you have obtained in question 1.1? Explain you answer in a short sentence.For the aqueous [Cu(NH3)4 ] 2+ complex kf =5.6 x1011 at25°C .Suppose equal volumes of 0.0062M Cu(NO3)2 solution and 0.88M NH3 solution are mixed. Calculate the equilibrium molarity of aqueous Cu2+ ion.Round your answer to 2 significant digits.Calculate the solubility (in g/L) of Aluminum sulfide (Al2S3) at 25°C {Ksp for Al2S3 is 1.5x10^-27}. 1) in pure water 2) in 0.10 M H2S solution and in 0.25M Al^+3 solution
- For the aqueous [HgCl4]2- complex Kf=5.0 x1015 at 25°C.Suppose equal volumes of 0.0074M Hg(NO3)2 solution and 0.84M NaCl solution are mixed. Calculate the equilibrium molarity of aqueous Hg 2+ ion.Round your answer to 2 significant digits.An aqueous solution of 43.80 mL of 0.240 mol L-1 NaCN is added to 43.80 mL of 0.240 mol L-1 Zn(NO3)2(aq) at 25oC. Determine the concentration (in mol L-1) of Zn(CN)42- in the solution once it reaches thermodynamic equilibrium at 25oC. Kf(Zn(CN)42-)=2.1×1019the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…
- the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…
- the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…the free energy for a reaction can be related to the equilibriumconstant through the formula below.K = e (-ΔG° / RT)Therefore if Kc for a reaction is known, Go can be determined, or vice versa. Furthermore, ifyou have the value for Go at two different temperatures, you can calculate H and S throughthe familiar equation for Gibbs energy below, since you have two unknowns but also twoequations.G = H – T SIn this lab you will be studying the solubility of borax (Na2B4O5(OH)4*8H2O), a slightly solublesodium salt, at two different temperatures. When solid borax is added to water, theequilibrium below is established.Na2B4O5(OH)4*8H2O (s) 2 Na+ (aq) + B4O5(OH)42- (aq) + 8 H2O(l)If you measure the concentrations for those substances that show up in the reaction quotient,then the Kc for the reaction at that temperature can be calculated. In this lab, theconcentration of borate ion (B4O5(OH)42-) in solution will be measured by titration with standardhydrochloric acid according to the…



