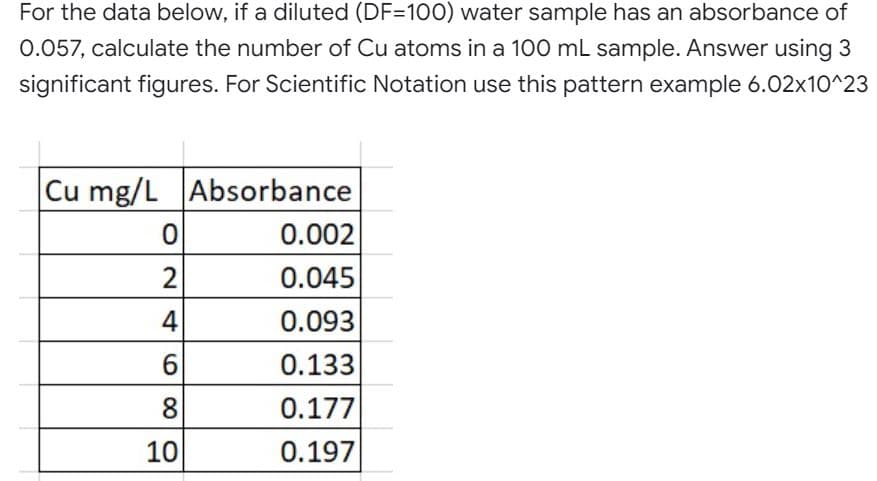
For the data below, if a diluted (DF=100) water sample has an absorbance of 0.057, calculate the number of Cu atoms in a 100 mL sample. Answer using 3 significant figures. For Scientific Notation use this pattern example 6.02x10^23 Cu mg/L Absorbance 0 0.002 2 0.045 0.093 0.133 0.177 0.197 460 8 10
Q: Two chemical species in this reaction can be classified as acids. Which are they? NH3 + H2O -->…
A:
Q: determine the sterocenters and R/S
A: Given: structure
Q: (R) (S) ОНС XIII
A: R/S configuration is given by CIP rule. Initially the priority is assigned to all the four groups…
Q: Which of the following statements is INCORRECT regarding ionization energy? a. Removal of electron…
A: Ionization energy can be defined as energy that is required to remove electron from an atom.
Q: EXPERIMENT Prepare 2 clear glasses. Label the glasses with letter A and B Put cold water in letter…
A: Chemical kinetics can be defined as the branch of chemistry that deals with rates of chemical…
Q: Consider the reaction of 2-bromo-2-methylpropane with water, shown below, to answer the following…
A:
Q: What element is produced by the alpha decay of 210Bi? Refer your answer to the following Periodic…
A:
Q: 1) What type of crystal will each of the following substances form in its solid-state? Choices to…
A: Ionic compounds have ions in their solid state. Metallic compounds have metal in solid state.…
Q: HCN is a monoprotic weak acid with a K, value of 4.90 x 10-10. Calculate the pH of a 6.50 x 10-6 M…
A:
Q: Consider the following energy diagram. D B Energy с E G Which step has the least endergonic AG*?…
A: Endergonic reactions may also be called an unfavorable reaction or nonspontaneous reaction.Since…
Q: Provide IUPAC names for the following structures. H₂C Br Sp Br H₂C Br a. CH3 b. Br Br
A: we have to determine the IUPAC name of the structures
Q: Draw the structure of the repeat unit of the polymer that results when this epoxide and this diamine…
A: Draw the polymer
Q: Select the reaction where work, w, is negative. O a. N₂(g) + 3H2(g)- 2NH3(g) O b. 2H₂(g) +…
A: Work done is negative when work is performed by the system.
Q: 7. Name the following compounds: A) CoCl₂ Name: B) P3N5 Name: C) (NH4)3P Name: Name: D) GeO₂
A: Different chemical compounds in inorganic chemistry have different names depending on the metallic…
Q: When the equation is Al + Br2 → AlBr3 is balanced, the coefficient for Al is______ A. 1 B. 2 C. 4…
A: Balanced chemical equation is the equation in which number of atoms on both sides of equation are…
Q: IR Spectrum (quid fm) 4000 3000 Lada 40 80 13C NMR Spectrum (100 0 MHz CDC), solution) 100 % of base…
A: Compound is 2- phenylethanol
Q: If 110.0 mL of 0.0030 MNa2SO4(aq) is saturated with CaSO4, how many grams of CaSO4 would be present…
A: Given: Concentration of Na2SO4 = 0.003 M And volume of solution = 110.0 mL = 0.110 L…
Q: Question is based on the following definitions. Plants can propagate, or reproduce themselves, by…
A: This reproductive form resembles bulb most closely as in that too by dividing plant starts growing…
Q: Determine the initial pH of a 15.5mL HCl solution that was titrated with 16.9mL of 0.075mol/L NaOH…
A: Sodium hydroxide reacts with hydrochloric acid, to form sodium chloride and water. The equation for…
Q: +1.40V +1.69V Au³+ Au+ Au* will spontaneously reduce to Au Au* will spontaneously oxidize to Au³+…
A:
Q: Classify the type of elementary step shown below. N=C Br CN A) proton transfer B) coordination C)…
A: Classification of type of elementary step in the given reaction.
Q: 2. If 25.0 mL of NaOH required 40.0 mL of H2SO4 in a titration and 25.0 mL of sulfuric acid were…
A:
Q: Which of the following arranges the groups in order of decreasing priority according to the sequence…
A: Given are some groups and we will have to arrange them in decreasing priority. We will be using the…
Q: h Consider this reaction when answering the following question(s): :0: :0: :0: acid H₂C CH3 CH3…
A: Organic compounds can be defined as the compounds that contain carbon and hydrogen atoms in a…
Q: 3. A student wishes to make a solution using barium phosphate. When he consulted the solubility…
A: Given that - Anion = Phosphate, PO43- Highly Soluble cations = Group 1 , NH4+ Low Soluble cations…
Q: What is being oxidized and reduced in this reaction? + CuSO FeSO 4(aq) + Cu(s) Fe (s) → 4(aq) A Fe…
A: What is oxidized and reduced in this reaction above--
Q: Airbags used in automobiles are inflated with nitrogen gas produced from the reaction of sodium…
A:
Q: 3 Cu + 8HNO3 -->3 Cu(NO3)2 + 2 NO + 4 H₂O In the above equation how many moles of water can be made…
A:
Q: All of the following statements regarding galvanic cells are TRUE EXCEPT: (A) Electrons flow from…
A: Galvanic cell is used to convert chemical energy stored to electrical energy as a result of chemical…
Q: Solid aluminum (Al)and chlorine (C1₂) gas react to form solid aluminum chloride (AIC13). Suppose you…
A:
Q: Identify the weak acid from the list. - HF - H2CO3 - H2SO2 - all of these
A: The species that increase the concentration of hydrogen ions or hydronium in an aqueous solution act…
Q: 1.2500 g sample of zinc oxide, 95.0% ZnO were treated with 50.00 mL of 1.1230 N sulfuric acid in the…
A:
Q: 4. Suggest a synthesis for the following from the indicated. starting material and any necessary…
A: “Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: Q1 If you known: So 0 SI-2 $2-6 S3 10 S4 14 Vo-0 VI-0.006 V2-0.01 V3-0.012 V4-0.014 Calculate Vmax,…
A:
Q: Which of the following is TRUE regarding Zn(NH3)4²+? O a. Zn(NH3)4²+ is colorless due to the filled…
A: According to the crystal field theory, in the presence of the tetrahedral ligand field, the five…
Q: Draw the skeletal (line-bond) structure of (1R, 3S)-1-bromo-3- isopropylcyclohexane.
A: (1R,3S)-1-bromo-3-isopropylcyclohexane.
Q: The boiling point of 1.00 kg of water at 749.2 mmHg is 99.60 °C. How much sucrose C12H22011 (molar…
A: Given, Mass of water solvent = 1.00 kg Boiling point of water solvent at 749.2 mmHg (Tb(water)) =…
Q: Question 7
A:
Q: Write the complete stepwise mechanism for the reaction below: (Non-anonymous question Ⓒ) OH HC1 OCH3…
A:
Q: Write the mechanism of the reaction of trans-2-butene with hydrogen bromide.
A: Given, Trans-2-butene + hydrogen bromide ------>
Q: 1. Fill in the blanks with the most appropriate term: A are the tells the story of a chemical…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: Identify the 'type' of organic reaction represented below. Put your answer in the box provided…
A: Substitution reaction involves replacing one atom/group by another atom/group. Addition reaction…
Q: 1. Write down the quantum numbers (n, I, and ms) that best describe the electron identified in the…
A: Here, we have to find the values of the quantum numbers (n, l, and ms) for the indicated electron.
Q: The amount of heat absorbed or evolved when specifically one mole of the substance is produced at…
A:
Q: 1. Calculate the mass of solid NaOH required to make 1.0L of a 0.50M NaOH solution. 2. Calculate the…
A:
Q: Considering the provided criteria, which set of compounds is ordered CORRECTLY? a.Decreasing normal…
A: Boiling point is temperature at which vapour pressure of liquid become equal to atmospheric…
Q: Consider the reaction: ICl(g) + Cl₂(g) → ICl₃(s). The ∆G° of the reaction is -17.09 kJ/mol.…
A: Recall the given reaction, ICl g + Cl2 g → ICl3 sThe ∆G°…
Q: C2H2O is the empirical formula for Ethanone. Asprin has the formula C9H8O4 how do I get this answer?
A:
Q: 513/variants/434513/take/11/ Question 14 In the following reaction, what is being reduced and what…
A: Oxidation reduction reaction (Redox reaction) It can be defined as the chemical reaction in which…
Q: For the reaction scheme below, label each reactant (identified as 'reactant 1-4') as either a…
A: Given; reaction mechanism.
Step by step
Solved in 2 steps with 1 images

- A 0.1 g amine-containing compound is dissolved in water then diluted to 100 mL. You subject it to spectroscopic analysis, in order to get the concentration of amine in this compound. Next, you get 1 mL of the previously diluted sample then dilute it again to 250 mL for measurement. Then, you fill 3/4 of a 1-cm cuvette with this diluted sample, and you run an analysis using an AAS. The recorded absorbance is 0.545 at 410 nm. What is the molecular weight of the compound? (The molar absorptivity is 1.23 x 104 cm-1 mol-1 L.)Caffeine (C8H10O2N4 • H2O) has an absorbance of 0.510 at 272 nm and 1 cm optical path in1 mg / 100 mL concentration solutions. A 2.5 g sample of soluble coffee is diluted withwater to 500 mL. Take 250 mL, add 25.0 mL of 0.1 N H2SO4 and dilute it to 500 mL. Theabsorbance at 272 nm resulting in 0.415. A) Calculate the grams of caffeine per kg of soluble coffee in the sample. Themolar mass of caffeine is 212 g / mol.A calibration curve of KCl conductivity (y-axis) vs concentration (mass percent, x-axis) is made. The equation of line of best fit is y = 578.3x + 0.380 and the R 2 is 0.9998. Based on this data what is the mass percent of an unknown KCl solution with a conductivity of 2,788 uS/cm? 6.55 % 6.12 % 4.14 % 3.98 % 4.82 %
- An unknown cobalt (II) chloride solution is measured to have an absorbance of 0.356. Calculate the molarity of the unknown cobalt (II) chloride solution using a spectrophotometer with a path length of 1.00 cm.A student was given a stock aluminum(III) solution with a concentration of 5.000 parts per million (ppm). (ppm are defined as mg/L for dilute aqueous solutions.) The student prepared five 100 mL standard solutions and an unknown as described in the procedure section of the experiment. The absorbance of each solution was read at 565 nm, using the blank to set zero absorbance. The results are tabulated below. Solution Volume of Al(III) stock solution used (mL) Absorbance 1 8.00 0.701 2 6.00 0.548 3 4.00 0.378 4 2.00 0.208 5 1.00 0.123 Unknown --- 0.534 3. What is your best estimate of the Al(III) concentration of the unknown sample in the cuvet based upon where its absorbance falls on the standard curve?From the following data, calculate the concentration of the analyte in the sample read at 700 nm: Absorbance of unknown sample = 0.807 Absorbance of a 130 mg/dl standard = 0.234 Do not answer in image format. Maintain accuracy and quality in your answer. Answer completely.
- Determine phosphate concentration of a water sample using the following information: Standard curve- slope: 1763.2 y-int: 0.011806 R^2: 0.99181 Absorbance of water sample: 0.013Solution. Vol. of A solution (mL) [methyl red], M Absorbance 1 10.00 _1.64 x10^-16_ _0.256_ 2 15.00 _2.45 x10^-16_. _0.373_ 3 20.00 _3.27 x10^-16_. _0.486_ 4 25.00 _4.09 x10^-16_. _0.620_ Plot a graph of the absorbance (y axis) as a function of the concentration of methyl red (x axis). What is the slope of the graph?The dye "Smoky Red" (SR) also reacts with NaOH(aq). You are in the lab and perform the following steps: Run1: 20 mL of SR stock solution (1.9 X 10-4 M) diluted to 100 mL 10 mL of NaOH stock solution (2.2 X 10-1 M) diluted to 100 mL at t=0, two above solutions are mixed SR Absorbance (Beer's Law constant = 1.2 X 105) is measured at 90 sec intervals Run2: 20 mL of SR stock solution diluted to 100 mL 20 mL of NaOH stock solution diluted to 100 mL at t=0, two above solutions are mixed SR Absorbance is measured at 60 sec intervals Reciprocal plot of SR absorbance gives q2 = 30.6 X 10-2 sec-1 The reaction of Smoky Red with sodium hydroxide solution is found to occur with a pseudo rate constant q1 of 7.3 X 10-2 sec-1. After Run 2 when volume of sodium hydroxide is doubled, q2 = 30.6 X 10-2 sec-1. Assuming the reaction is second order with respect to the dye, what is n for this reaction (round to the nearest whole number)?
- 1. How would you find the solution for this dilution and absorbance problem? What would the set-up for solving this look like? (A = 0.0252C(ppm) - 0.0162)The glucose content of samples can be determined using Nelson’s test. The equation of the line for the standard curve prepared for varying concentrations of glucose is y = 3.0x + 0.2. Based on this information, determine the glucose concentration (in M) of a mango preserve having an absorbance reading of 0.891.How many grams of NaAsO, should be weighed out to prepare a stock solution of 100 mg/L in 500 mL deionized water. Use the stock solution to prepare 4 different standard solution that can be used to prepare an analytical calibration curve to measure the concentration of 750 sample in the concentration range of 10-35 mg/L. Draw a proposed calibration curve (based on the results you achieved).

