For the following compound: OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Сон Mechanism B: O-H of of :O-H Mechanism C: :OH O-H Mechanism D: :OH H-OH₂ OH H-O-H :OH H.. HO-H + H.. 1 H HH
For the following compound: OH Draw a mechanism for the tautomerization process under ACIDIC conditions: Mechanism A: Сон Mechanism B: O-H of of :O-H Mechanism C: :OH O-H Mechanism D: :OH H-OH₂ OH H-O-H :OH H.. HO-H + H.. 1 H HH
Chapter17: Alcohols And Phenols
Section17.SE: Something Extra
Problem 60AP: Benzvl chloride can be converted into benzaldehvde by treatment with nitromethane and base. The...
Related questions
Question
Please answer the following question correctly and re do it to confirm
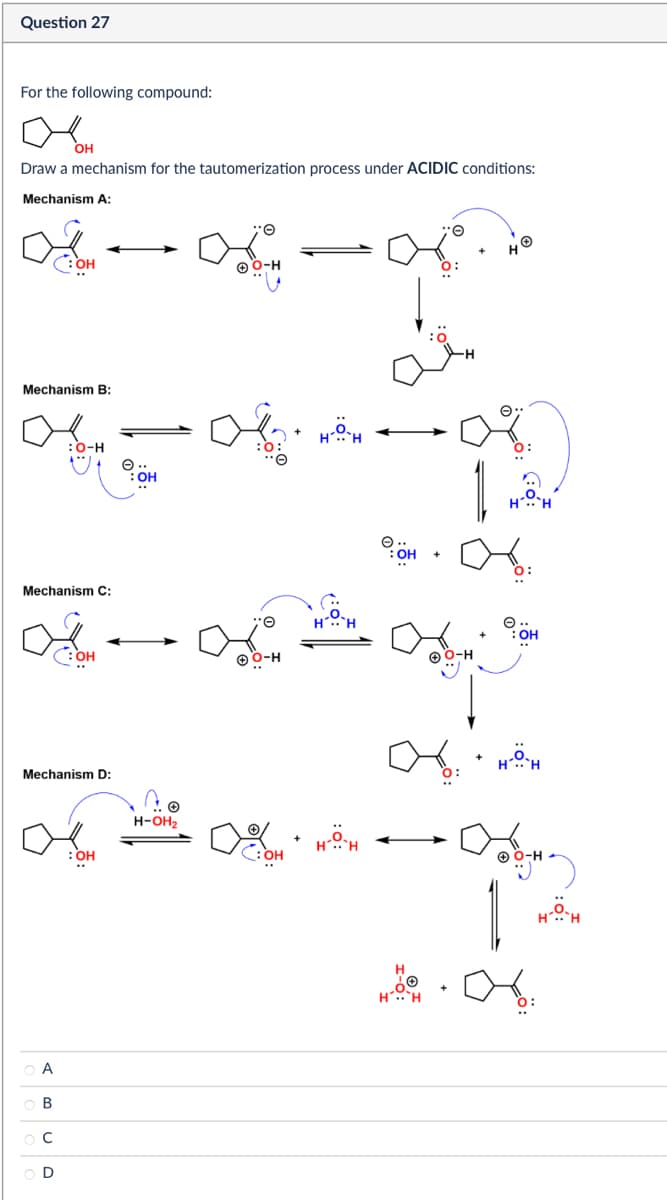
Transcribed Image Text:Question 27
For the following compound:
ол
OH
Draw a mechanism for the tautomerization process under ACIDIC conditions:
Mechanism A:
HO:
06
H-O.
Mechanism B:
HO:
H-O:
Mechanism C:
но:
70-20
Mechanism D:
:OH
H-OH₂
ABCD
OA
ос
H.. H
H.H
Θ
H.. H
C:OH
HO:
1
어
H.. H
O..
HO:
O-H
H.. H
+
H..H
H.. H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

