For the following questions, use the following diagrams. Uncatalyzed Reaction Catalyzed Reaction 400 400 300 300 reactants reactants 200 200 100 100 products products Reaction coordinate Reaction coordinate (a) What is the value of the forward activation energy of the uncatalyzed reaction? (b) What is the value of the reverse activation energy of the catalyzed reaction? (c) What is the overall enthalpy (AH) of the forward reaction? Potential energy (kJ)
For the following questions, use the following diagrams. Uncatalyzed Reaction Catalyzed Reaction 400 400 300 300 reactants reactants 200 200 100 100 products products Reaction coordinate Reaction coordinate (a) What is the value of the forward activation energy of the uncatalyzed reaction? (b) What is the value of the reverse activation energy of the catalyzed reaction? (c) What is the overall enthalpy (AH) of the forward reaction? Potential energy (kJ)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 72QAP: Consider a reaction in which E a=129 kJ and H=29 kJ. In the presence of a catalyst, the activation...
Related questions
Question
Problem attached
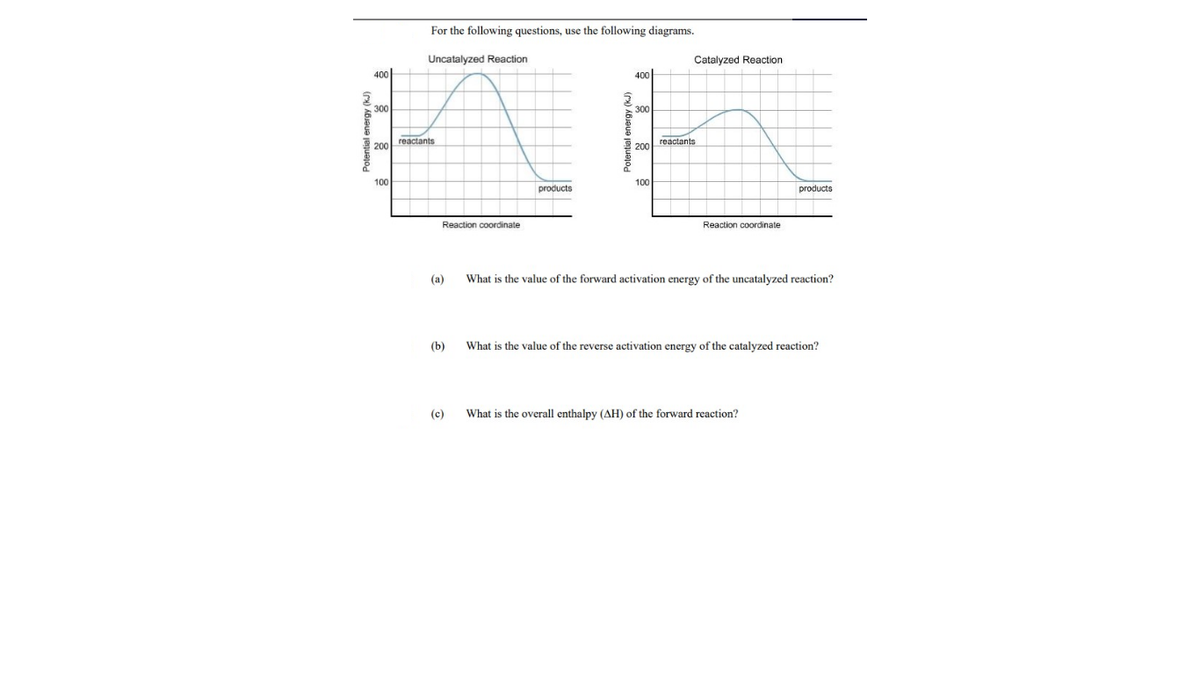
Transcribed Image Text:For the following questions, use the following diagrams.
Uncatalyzed Reaction
Catalyzed Reaction
400
400
300
300
reactants
reactants
200
200
100
100
products
products
Reaction coordinate
Reaction coordinate
(a)
What is the value of the forward activation energy of the uncatalyzed reaction?
(b)
What is the value of the reverse activation energy of the catalyzed reaction?
(c)
What is the overall enthalpy (AH) of the forward reaction?
Potential energy (kJ)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning