For the following reaction, 3.50 grams of chlorine gas are mixed with excess phosphorus (P). The reaction yields 3.69 grams of phosphorus trichloride. phosphorus (P)(s)+ chlorine ( g) phosphorus trichloride (1) What is the theoretical yield of phosphorus trichloride ? grams What is the percent yield for this reaction ? %
For the following reaction, 3.50 grams of chlorine gas are mixed with excess phosphorus (P). The reaction yields 3.69 grams of phosphorus trichloride. phosphorus (P)(s)+ chlorine ( g) phosphorus trichloride (1) What is the theoretical yield of phosphorus trichloride ? grams What is the percent yield for this reaction ? %
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter6: Chemical Calculations: Formula Masses, Moles, And Chemical Equations
Section: Chapter Questions
Problem 6.84EP: In an experiment designed to produce calcium oxide by the chemical reaction 2Ca + O2 2CaO 177.2 g...
Related questions
Question
One question. I split them
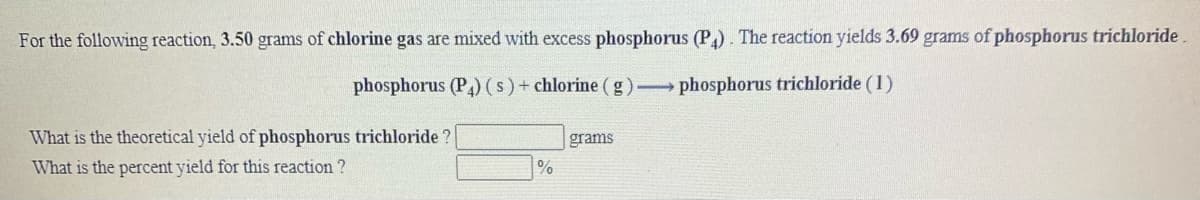
Transcribed Image Text:For the following reaction, 3.50 grams of chlorine gas are mixed with excess phosphorus (P). The reaction yields 3.69 grams of phosphorus trichloride
phosphorus (P) (s)+ chlorine (g) phosphorus trichloride (1)
What is the theoretical yield of phosphorus trichloride ?
grams
What is the percent yield for this reaction ?

Transcribed Image Text:For the following reaction, 6.12 grams of copper are mixed with excess silver nitrate. The reaction yields 15.6 grams of copper(II) nitrate.
silver nitrate (aq) + copper (s) copper(II) nitrate (aq) + silver (s)
What is the theoretical yield of copper(II) nitrate ?
grams
What is the percent yield of copper(II) nitrate ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning