For the reaction 2A+ 3B → 4C + 5D, the rate of the reaction in terms of AA would be written as which of the following? Why? a. -AA/At .-1/2 ΔΑΔr c. +AA/At d.+1/2 ΔΑΔ e.-2 ΔΑ/Δ
For the reaction 2A+ 3B → 4C + 5D, the rate of the reaction in terms of AA would be written as which of the following? Why? a. -AA/At .-1/2 ΔΑΔr c. +AA/At d.+1/2 ΔΑΔ e.-2 ΔΑ/Δ
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter12: Kinetics
Section: Chapter Questions
Problem 27E: For the reaction AB+C, the following data were obtained at 30 C: [A] (M) 0.230 0.356 0.557 Rate...
Related questions
Question
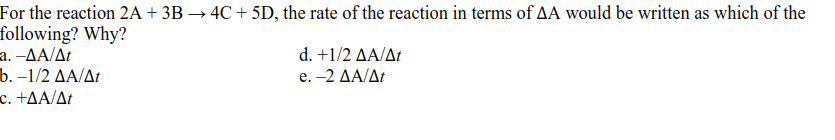
Transcribed Image Text:For the reaction 2A + 3B → 4C + 5D, the rate of the reaction in terms of AA would be written as which of the
following? Why?
a. -AA/At
b.-1/2 ΔΑΔr
c. +AA/At
d.+1/2 ΔΑΔ
e.-2 ΔΑ/Δt

Transcribed Image Text:A scientist conducts an experiment to determine the rate of the following reaction:
N2(g) + O2(g) → 2 NO(g)
If the initial concentration of N2 was 0.500 M and the concentration of N, was 0.450 M after 0.100 s, what is the
rate of the reaction?
a. 0.500 M/s
d. 10.0 M/s
b. 1.00 M/s
e. 0.250 M/s
c. 5.00 M/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
