Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0026 M·s : 2 HI (g) - H, (g) +L, (g) Suppose a 5.0 L flask is charged under these conditions with 150. mmol of hydrogen iodide. How much is left 3.0 s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0026 M·s : 2 HI (g) - H, (g) +L, (g) Suppose a 5.0 L flask is charged under these conditions with 150. mmol of hydrogen iodide. How much is left 3.0 s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 3RQ: One experimental procedure that can be used to determine the rate law of a reaction is the method of...
Related questions
Question
Please write in correct significance figures
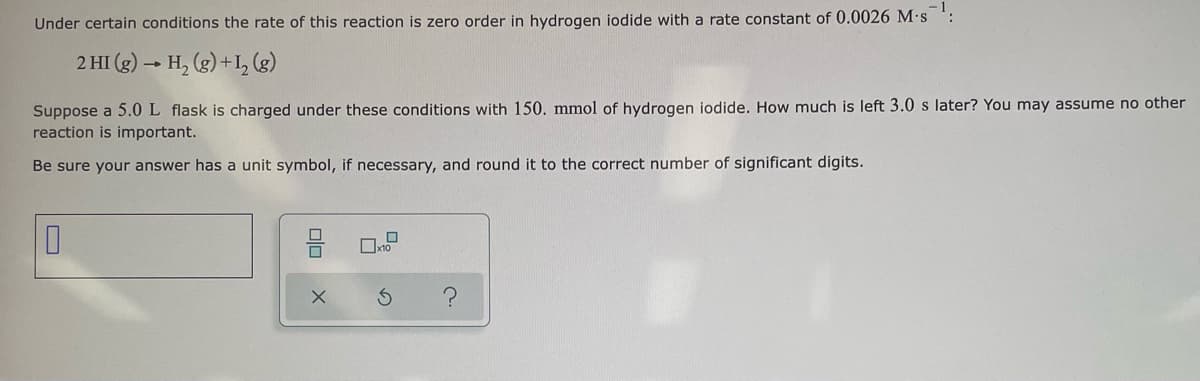
Transcribed Image Text:Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0026 M.s :
2 HI (g) → H, (g) +I, (g)
Suppose a 5.0 L flask is charged under these conditions with 150. mmol of hydrogen iodide. How much is left 3.0 s later? You may assume no other
reaction is important.
Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
미□
Expert Solution
Step 1
The rate of the zero-order reaction does not depend on the concentration of the reactant. For the zero-order reaction, the rate constant is given by:
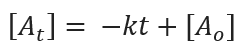
Where [Ao] = initial concentration of the reactant
[At] = concentration of the reactant after t time
k = rate constant of the reaction = 0.0026 M.s-1
t = time = 3.0 s
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning