For the reaction HCI (aq) + CO2 (aq) = Cl¯ (aq) + HCO3 (aq) identify one change that would shift the equilibrium to reactants. adding HCI O adding CO2- O removing CO- removing Cl
For the reaction HCI (aq) + CO2 (aq) = Cl¯ (aq) + HCO3 (aq) identify one change that would shift the equilibrium to reactants. adding HCI O adding CO2- O removing CO- removing Cl
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 9QAP: When writing a chemical equation for a reaction that comes to equilibrium. how do we indicate...
Related questions
Question
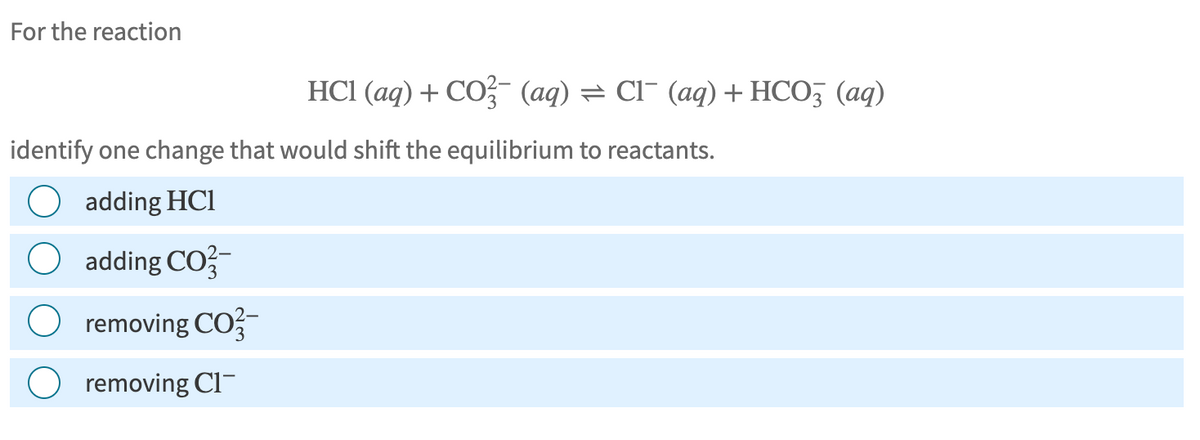
Transcribed Image Text:For the reaction
HCI (aq) + CO2 (aq) = Cl¯ (aq) + HCO3 (aq)
identify one change that would shift the equilibrium to reactants.
adding HCI
O adding CO2-
O removing CO-
removing Cl
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning