For the treatment of his indigestion, Tin must drink a salt solution with a pH between 8.00 to 8.50. Tan, Tin's friend, wanted to give Tin a 0.895 mM solution of a water-soluble salt generated from the ions K+ and C3H2O42-. a. Write the chemical formula of the salt offered by Tan. b. What is the hydrolysis reaction for the salt solution offered by Tan. c. What is the pH of the salt solution? (2 decimal places)
For the treatment of his indigestion, Tin must drink a salt solution with a pH between 8.00 to 8.50. Tan, Tin's friend, wanted to give Tin a 0.895 mM solution of a water-soluble salt generated from the ions K+ and C3H2O42-. a. Write the chemical formula of the salt offered by Tan. b. What is the hydrolysis reaction for the salt solution offered by Tan. c. What is the pH of the salt solution? (2 decimal places)
Chapter1: Numerals And Fractions
Section: Chapter Questions
Problem 1RP
Related questions
Question
For the treatment of his indigestion, Tin must drink a salt solution with a pH between 8.00 to 8.50. Tan, Tin's friend, wanted to give Tin a 0.895 mM solution of a water-soluble salt generated from the ions K+ and C3H2O42-.
a. Write the chemical formula of the salt offered by Tan.
b. What is the hydrolysis reaction for the salt solution offered by Tan.
c. What is the pH of the salt solution? (2 decimal places)
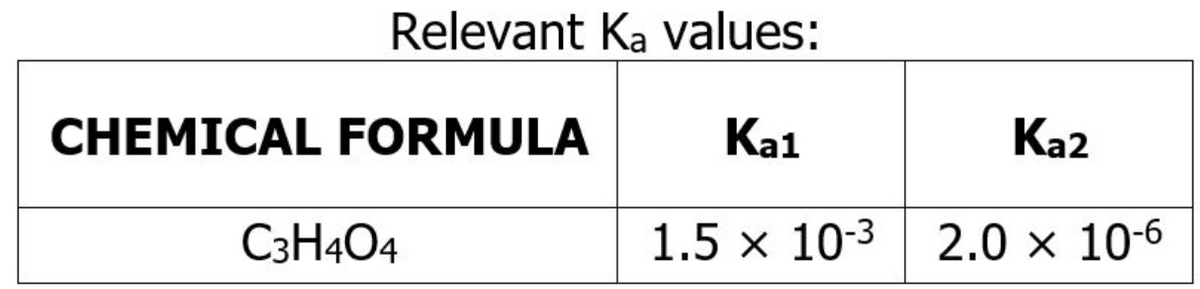
Transcribed Image Text:Relevant Ka values:
CHEMICAL FORMULA
Ka1
Ka2
C3H404
1.5 x 10-3 2.0 × 10-6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

