For which of the following processes is the equation AS = qrev /T valid? 4. any reversible expansion of an ideal gas. isothermal irreversible compression of a non-ideal gas. constant pressure heating of a liquid. isothermal vaporization of a liquid. isothermal sublimation of a solid to a non-ideal vapor. 1. 2. 3. 4. 5. 1 & 3 only B) 1, 4 & 5 only E) 4 & 5 only C) 2, 3 & 4 only A) D) 2, 4 & 5 only
For which of the following processes is the equation AS = qrev /T valid? 4. any reversible expansion of an ideal gas. isothermal irreversible compression of a non-ideal gas. constant pressure heating of a liquid. isothermal vaporization of a liquid. isothermal sublimation of a solid to a non-ideal vapor. 1. 2. 3. 4. 5. 1 & 3 only B) 1, 4 & 5 only E) 4 & 5 only C) 2, 3 & 4 only A) D) 2, 4 & 5 only
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.32E: Many compressed gases come in large,heavy metal cylindersthat are so heavy that they need a special...
Related questions
Question
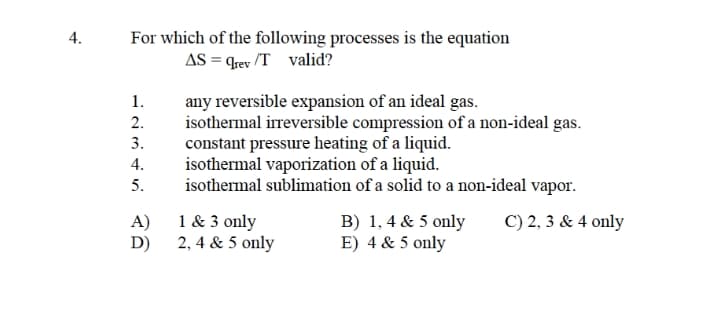
Transcribed Image Text:For which of the following processes is the equation
AS = qrev /T valid?
any reversible expansion of an ideal gas.
isothermal irreversible compression of a non-ideal gas.
constant pressure heating of a liquid.
isothermal vaporization of a liquid.
isothermal sublimation of a solid to a non-ideal vapor.
1.
2.
3.
4.
5.
1 & 3 only
A)
2, 4 & 5 only
D)
B) 1, 4 & 5 only
E) 4 & 5 only
C) 2, 3 & 4 only
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,