formula but different arrangements of atoms. Use Table 8.3 to estimate AH for each of the following gas-phase isomer- ization reactions and indicate which isomer has the lower enthalpy H H I I (а) Н—С—С—0-н — Н-С-0-С-Н Нн Ethanol Dimethyl ether но — Н—С—С—Н (b) Н——С-H T I нн н Acetaldehyde Ethylene oxide Н, Н. н H HHH H I| С—С—С—С-С—Н (c) H Н H н' Pentadiene Cyclopentene Н Н Н-С—NEC — Н—С-СEN (d) Н Н Methyl isocyanide Acetonitrile | Strengths and Lengths 8.8 of Covalent Bonds The stability of a molecule is related to the strengths of its covalent bonds. We saw in Chapter 5 that we can measure the average bond enthalpy of many single bonds (Table 5.4). Table 5.4 is repeated here as Table 8.3. As you might expect, Table 8.3 shows that multiple bonds are generally stronger than single bonds TABLE 8.3 Average Bond Enthalpies (k)J/mol) Single Bonds 413 N-H 391 о--н F-F 463 155 С-н N-N 163 348 О-О 146 С-с N-O 201 293 О-F 190 Cl-F 253 C-N N-F О— СI 272 358 203 Cl-Cl 242 С-о N-CI 200 О-1 485 234 С-F N-Br 243 328 Br-F 237 C-CI 276 S-H 339 Br-Cl 218 С-Br Н--Н 436 SF 240 327 Br-Br 193 С-1 Н-F 567 S-Cl 259 253 С-S Н-СI 431 S-Br 218 I-CI 208 Н- Br 366 323 S-S 266 I--Br Si-H 175 Н-I 299 226 Si-Si I-I 151 301 Si-C 368 Si-O 464 Si-Cl Multiple Bonds C=C 614 N=N 418 O=O 495 C=C 839 N=N 941 C=N 615 N- O 607 S=O 523 C=N 891 S=S 418 C=O 799 C O 1072
formula but different arrangements of atoms. Use Table 8.3 to estimate AH for each of the following gas-phase isomer- ization reactions and indicate which isomer has the lower enthalpy H H I I (а) Н—С—С—0-н — Н-С-0-С-Н Нн Ethanol Dimethyl ether но — Н—С—С—Н (b) Н——С-H T I нн н Acetaldehyde Ethylene oxide Н, Н. н H HHH H I| С—С—С—С-С—Н (c) H Н H н' Pentadiene Cyclopentene Н Н Н-С—NEC — Н—С-СEN (d) Н Н Methyl isocyanide Acetonitrile | Strengths and Lengths 8.8 of Covalent Bonds The stability of a molecule is related to the strengths of its covalent bonds. We saw in Chapter 5 that we can measure the average bond enthalpy of many single bonds (Table 5.4). Table 5.4 is repeated here as Table 8.3. As you might expect, Table 8.3 shows that multiple bonds are generally stronger than single bonds TABLE 8.3 Average Bond Enthalpies (k)J/mol) Single Bonds 413 N-H 391 о--н F-F 463 155 С-н N-N 163 348 О-О 146 С-с N-O 201 293 О-F 190 Cl-F 253 C-N N-F О— СI 272 358 203 Cl-Cl 242 С-о N-CI 200 О-1 485 234 С-F N-Br 243 328 Br-F 237 C-CI 276 S-H 339 Br-Cl 218 С-Br Н--Н 436 SF 240 327 Br-Br 193 С-1 Н-F 567 S-Cl 259 253 С-S Н-СI 431 S-Br 218 I-CI 208 Н- Br 366 323 S-S 266 I--Br Si-H 175 Н-I 299 226 Si-Si I-I 151 301 Si-C 368 Si-O 464 Si-Cl Multiple Bonds C=C 614 N=N 418 O=O 495 C=C 839 N=N 941 C=N 615 N- O 607 S=O 523 C=N 891 S=S 418 C=O 799 C O 1072
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter19: Eas: Electrophilic Aromatic Substitution
Section: Chapter Questions
Problem 19E
Related questions
Question
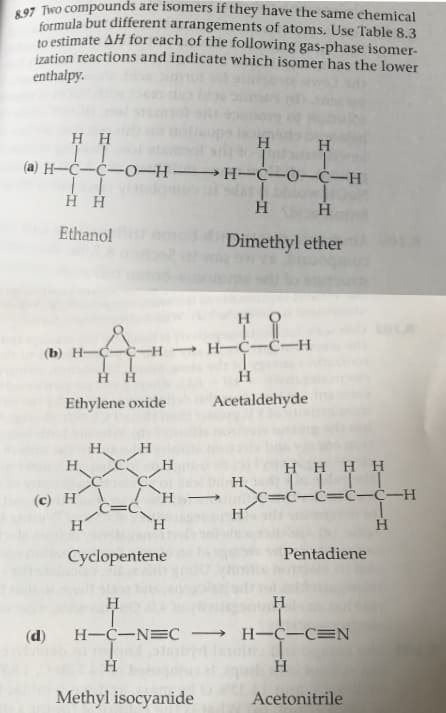
Transcribed Image Text:formula but different arrangements of atoms. Use Table 8.3
to estimate AH for each of the following gas-phase isomer-
ization reactions and indicate which isomer has the lower
enthalpy
H H
I I
(а) Н—С—С—0-н — Н-С-0-С-Н
Нн
Ethanol
Dimethyl ether
но
— Н—С—С—Н
(b) Н——С-H
T I
нн
н
Acetaldehyde
Ethylene oxide
Н,
Н.
н
H HHH
H I|
С—С—С—С-С—Н
(c) H
Н
H
н'
Pentadiene
Cyclopentene
Н
Н
Н-С—NEC — Н—С-СEN
(d)
Н
Н
Methyl isocyanide
Acetonitrile
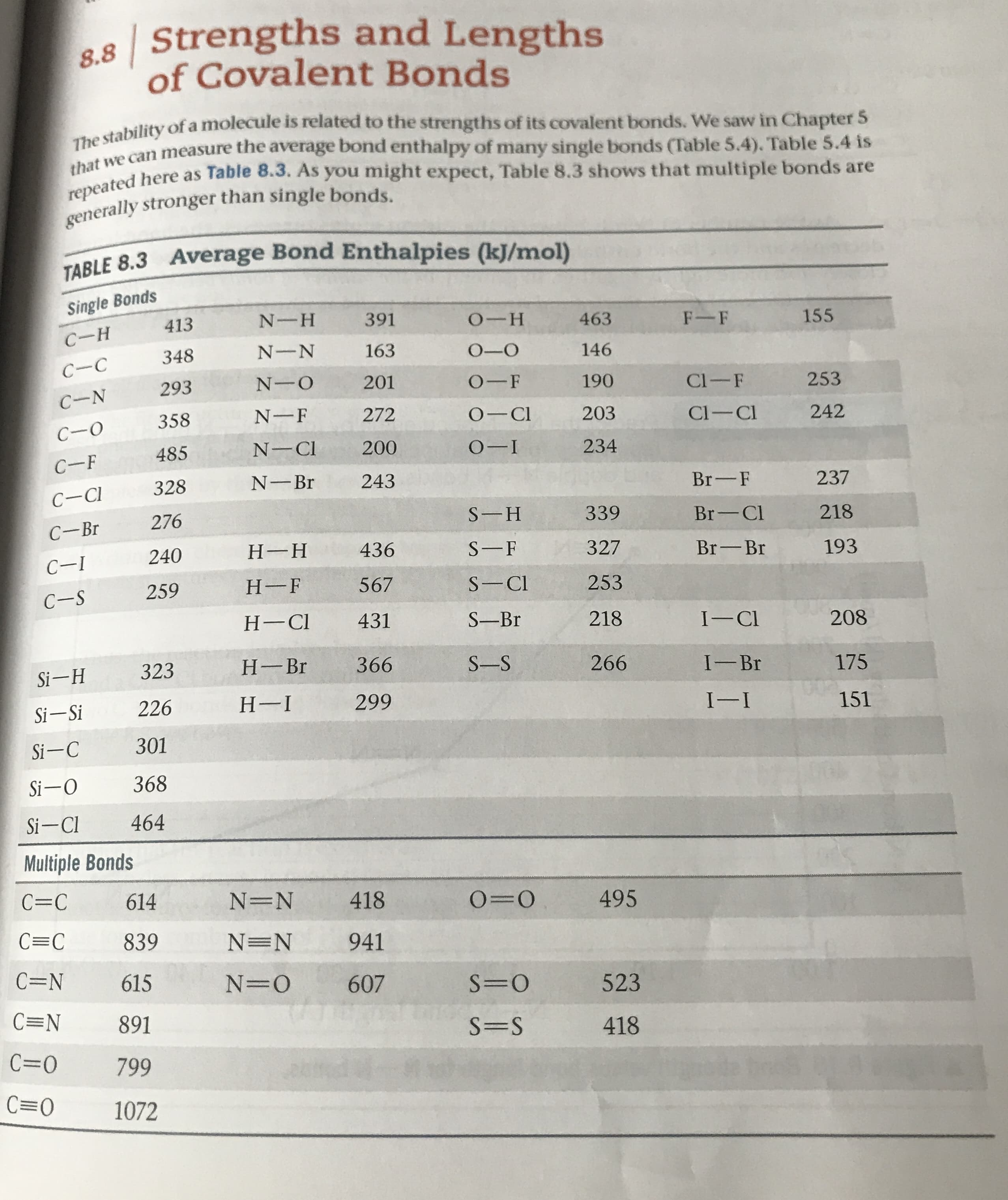
Transcribed Image Text:| Strengths and Lengths
8.8
of Covalent Bonds
The stability of a molecule is related to the strengths of its covalent bonds. We saw in Chapter 5
that we can measure the average bond enthalpy of many single bonds (Table 5.4). Table 5.4 is
repeated here as Table 8.3. As you might expect, Table 8.3 shows that multiple bonds are
generally stronger than single bonds
TABLE 8.3 Average Bond Enthalpies (k)J/mol)
Single Bonds
413
N-H
391
о--н
F-F
463
155
С-н
N-N
163
348
О-О
146
С-с
N-O
201
293
О-F
190
Cl-F
253
C-N
N-F
О— СI
272
358
203
Cl-Cl
242
С-о
N-CI
200
О-1
485
234
С-F
N-Br
243
328
Br-F
237
C-CI
276
S-H
339
Br-Cl
218
С-Br
Н--Н
436
SF
240
327
Br-Br
193
С-1
Н-F
567
S-Cl
259
253
С-S
Н-СI
431
S-Br
218
I-CI
208
Н- Br
366
323
S-S
266
I--Br
Si-H
175
Н-I
299
226
Si-Si
I-I
151
301
Si-C
368
Si-O
464
Si-Cl
Multiple Bonds
C=C
614
N=N
418
O=O
495
C=C
839
N=N
941
C=N
615
N- O
607
S=O
523
C=N
891
S=S
418
C=O
799
C O
1072
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
