Formulas for lonic Compounds 1. Write the chemical formula for the lonic compounds made from the following elements Elements potassium and iodine banum and chionine sodium and oxygen aluminum and nitrogen lithium and sulfur magnesium and oxygen calcium and chlorine boron and chlorine beryllium and sulfur Metal ion Non-metal ion Chemical Formula Name
Formulas for lonic Compounds 1. Write the chemical formula for the lonic compounds made from the following elements Elements potassium and iodine banum and chionine sodium and oxygen aluminum and nitrogen lithium and sulfur magnesium and oxygen calcium and chlorine boron and chlorine beryllium and sulfur Metal ion Non-metal ion Chemical Formula Name
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter3: Chemical Bonds
Section: Chapter Questions
Problem 3.117P
Related questions
Question
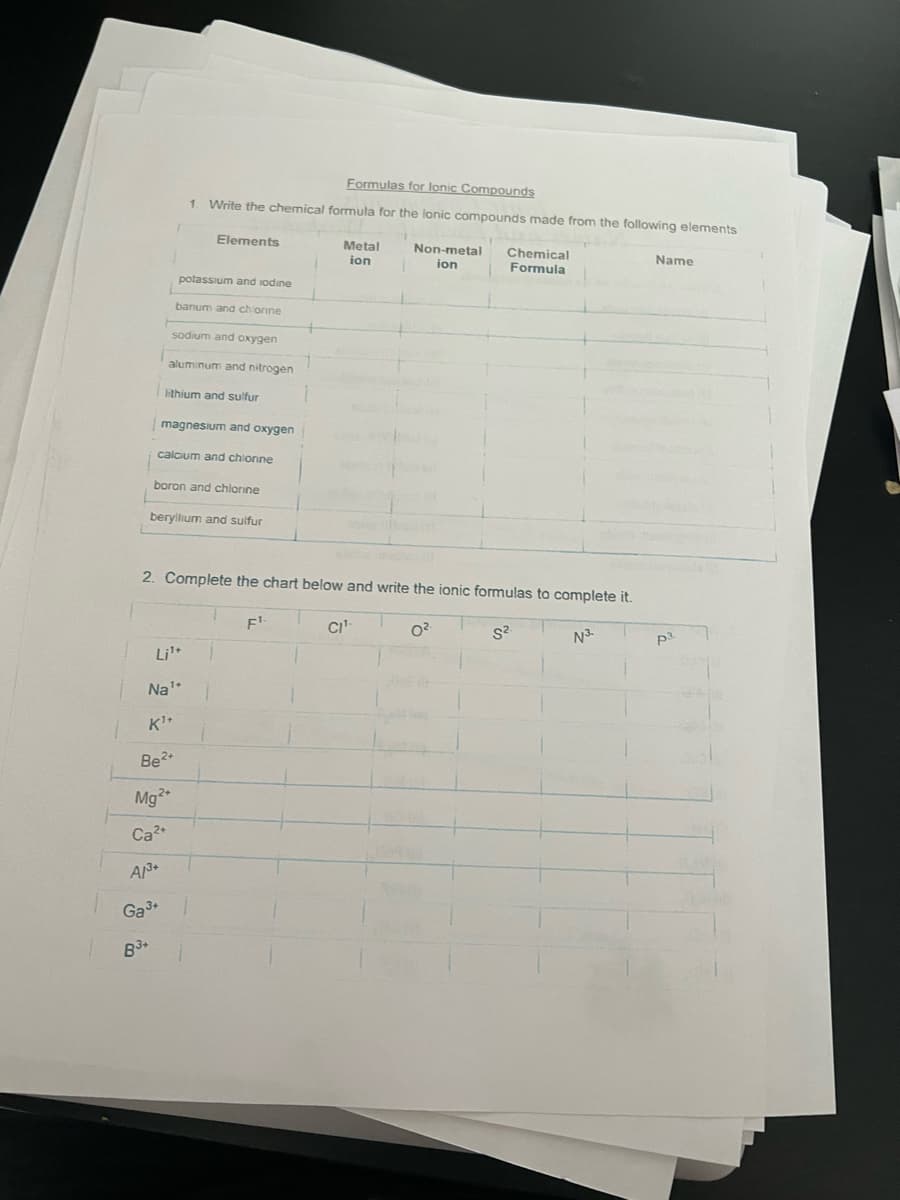
Transcribed Image Text:Formulas for lonic Compounds
1. Write the chemical formula for the lonic compounds made from the following elements
Elements
potassium and iodine
banum and chlorine
sodium and oxygen
aluminum and nitrogen
lithium and sulfur
magnesium and oxygen
calcium and chlorine
boron and chlorine
beryllium and sulfur
Li¹+
Na¹+
1+
K¹+
Be²+
Mg²+
Ca²+
AM³+
Ga³+
B3+
2. Complete the chart below and write the ionic formulas to complete it.
0².
S².
N³-
Metal Non-metal
ion 1 ion
F¹.
Chemical
Formula
CI¹-
Name
p3.
OJAM

Transcribed Image Text:Ionic Compounds:
Names and Formulas Worksheet
1. Write the formulas for the following compounds.
(a) magnesium oxide
(b) sodium fluoride
(c) aluminum nitride
(d) potassium sulfide
(e) lithium iodide
(f) calcium bromide
(g) beryllium oxide
(h) nickel chloride
(i) magnesium nitride
(j) aluminum sulfide
2. Write the names for the following compounds.
(a) Li₂O
(b) AICI,
(c) MgS
(d) Ca0
(e) KBr
(f) BeF
(g) Na N
(h) Al₂0₁
(i) CuCl₂
(j) Febr,
(k) copper(1) bromid
(1) tin(II) iodide
(m) iron(II) chloride
(n) calcium phosphide
(o) lead(II) oxide
(p) lead(IV) fluoride
(q) tin(IV) bromide
(r) copper(II) sulfide
(s) iron(II) oxide
(t) calcium nitride
(k) PbS
(1) SnO₂
(m) Na₂S
(n) Mg3P 2
(0) NiO
(p) Cul
(q) PbCl₂
(r) FeP
(s) CaF₂
(t) K,P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning