Four test tubes containing different hydrocarbons: acetone, benzoic acid, cyclohexane, and ethylamine were tested for solubility with different solvents: H2O, dilute HCl solution, and a strong solution of NaOH. The results obtained from the solubility test were tabulated below. Determine the content of each test tube A, B, C, and D.
Four test tubes containing different hydrocarbons: acetone, benzoic acid, cyclohexane, and ethylamine were tested for solubility with different solvents: H2O, dilute HCl solution, and a strong solution of NaOH. The results obtained from the solubility test were tabulated below. Determine the content of each test tube A, B, C, and D.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 59QAP: An aqueous solution of LiX is prepared by dissolving 3.58 g of the electrolyte in 283 mL of...
Related questions
Question
100%
Four test tubes containing different hydrocarbons: acetone, benzoic acid, cyclohexane, and ethylamine were tested for solubility with different solvents: H2O, dilute HCl solution, and a strong solution of NaOH. The results obtained from the solubility test were tabulated below.
Determine the content of each test tube A, B, C, and D.
Choices:
A. Cyclohexane
B. Ethylamine
C. Benzoic Acid
D. Acetone
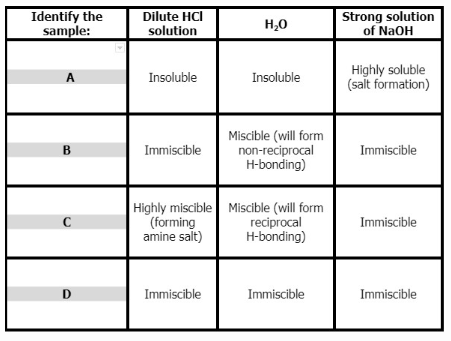
Transcribed Image Text:Identify the
sample:
Dilute HCI
solution
Strong solution
of NaOH
H,0
Highly soluble
(salt formation)
A
Insoluble
Insoluble
Miscible (will form
non-reciprocal
H-bonding)
B
Immiscible
Immiscible
Highly miscible Miscible (will form
(forming
amine salt)
reciprocal
H-bonding)
Immiscible
Immiscible
Immiscible
Immiscible
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning