Fri 3:26 PM istory Bookmarks Window Help A mcphs.blackboard.com anxiety statistics in high school... + Depression Statistics - Depress... DrugFacts: Marijuana | National... Take Test: Exam 4-2020SP.B... EasyBib: Free Bibliography Gen. rences to use for paper. - G.. Remaining Time: 56 minutes, 56 seconds. * Question Completion Status: Question 2 of 20 A Moving to another question will save this response. 5 points Save Answer Question 2 A 1.10 g sample of octane, C8H18, is combusted in a bomb calorimeter and the temperature rises by 62.0 °C. Given a Ccal value of 850.6 J/°C, determine AUrxn per mol of octane. -47.9 kJ/mol 52.7 kJ/mol -62.0 kJ/mol 5270 kJ/mol -5480 kJ/mol Question 2 of 20> LA Moving to another question will save this response. APR tv 10 MacBook Air DD 80 D00 DOO 888 F7 F8 F9 F10 F11 F12 F2 F3 F4 F5 F6 F1 ! %23 24 & 1 4 8. 9. delete { } Y [ 1 %3D return + || く
Fri 3:26 PM istory Bookmarks Window Help A mcphs.blackboard.com anxiety statistics in high school... + Depression Statistics - Depress... DrugFacts: Marijuana | National... Take Test: Exam 4-2020SP.B... EasyBib: Free Bibliography Gen. rences to use for paper. - G.. Remaining Time: 56 minutes, 56 seconds. * Question Completion Status: Question 2 of 20 A Moving to another question will save this response. 5 points Save Answer Question 2 A 1.10 g sample of octane, C8H18, is combusted in a bomb calorimeter and the temperature rises by 62.0 °C. Given a Ccal value of 850.6 J/°C, determine AUrxn per mol of octane. -47.9 kJ/mol 52.7 kJ/mol -62.0 kJ/mol 5270 kJ/mol -5480 kJ/mol Question 2 of 20> LA Moving to another question will save this response. APR tv 10 MacBook Air DD 80 D00 DOO 888 F7 F8 F9 F10 F11 F12 F2 F3 F4 F5 F6 F1 ! %23 24 & 1 4 8. 9. delete { } Y [ 1 %3D return + || く
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Transcribed Image Text:Fri 3:26 PM
istory Bookmarks Window Help
A mcphs.blackboard.com
anxiety statistics in high school... +
Depression Statistics - Depress...
DrugFacts: Marijuana | National...
Take Test: Exam 4-2020SP.B...
EasyBib: Free Bibliography Gen.
rences to use for paper. - G..
Remaining Time: 56 minutes, 56 seconds.
* Question Completion Status:
Question 2 of 20
A Moving to another question will save this response.
5 points
Save Answer
Question 2
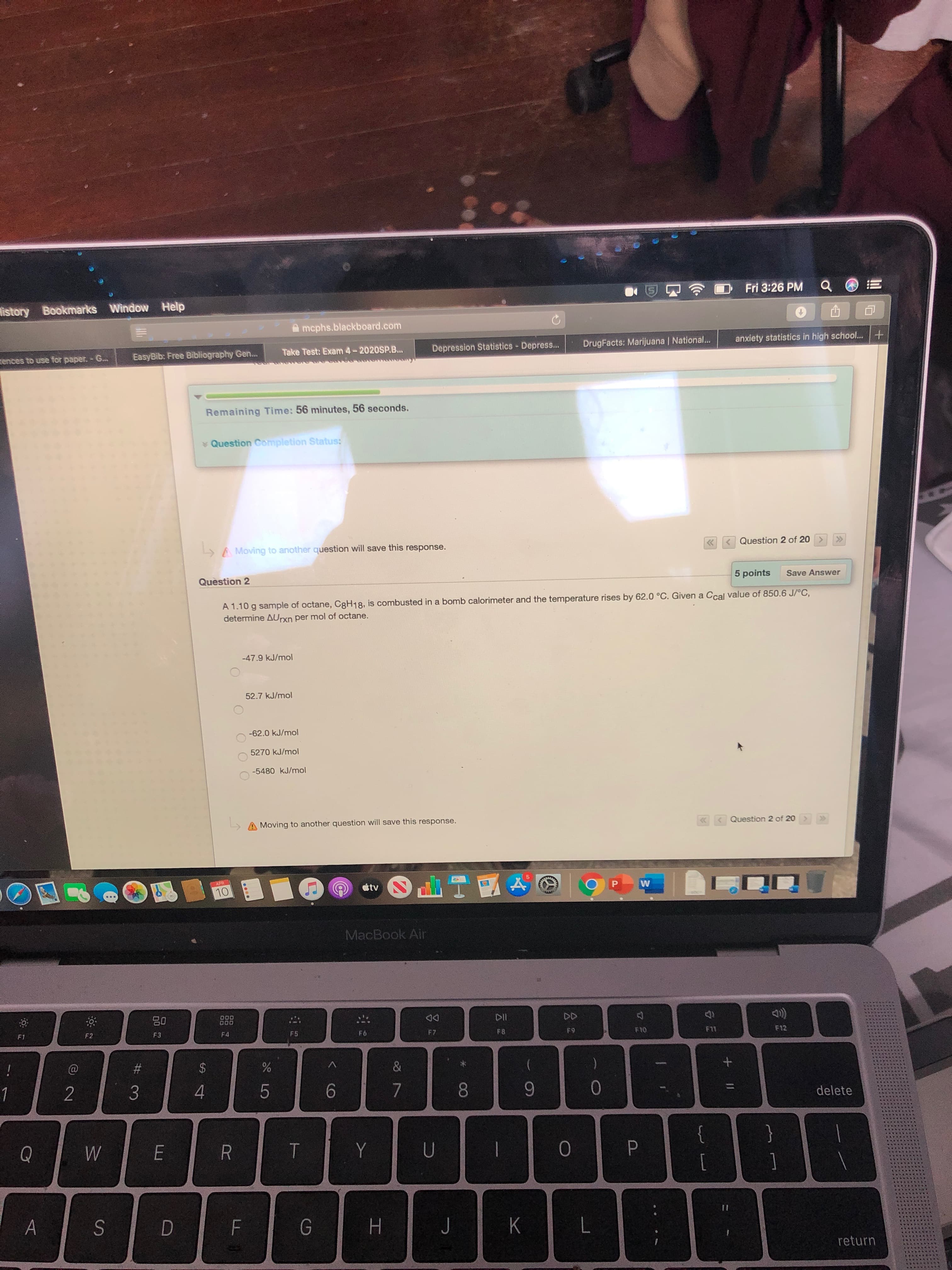
A 1.10 g sample of octane, C8H18, is combusted in a bomb calorimeter and the temperature rises by 62.0 °C. Given a Ccal value of 850.6 J/°C,
determine AUrxn per mol of octane.
-47.9 kJ/mol
52.7 kJ/mol
-62.0 kJ/mol
5270 kJ/mol
-5480 kJ/mol
Question 2 of 20>
LA Moving to another question will save this response.
APR
tv
10
MacBook Air
DD
80
D00
DOO
888
F7
F8
F9
F10
F11
F12
F2
F3
F4
F5
F6
F1
!
%23
24
&
1
4
8.
9.
delete
{
}
Y
[
1
%3D
return
+ ||
く
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning