New tab /gastate.view.usg.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame auto.d21?ou=D1995113&isprv=&drc%3D0&qi=2970873&cfql=D0&dnb Final Exam (1151Lab) Time Limit: 1:00:00 Time Left:0:48:39 Sierra Reynolds: Attempt 1 Page 1: Previous Page Next Page Page 8 of 24 Question 8 (1 point) When magnesium metal is placed into hydrochloric acid, vigorous bubbling is observed. Which of the following is the Page 2: equation for this reaction? Remember, your answer must use correct chemical formulas of the reactants and products. Mg + 2HCI MgCl, + H, Mg + H2CI H2 + MgCl Page 3: Mg + Cl2 > MgCl2 Mg + HCI -> MgCl Page 4: Page 8 of 24 Previous Page Next Page Page 5; Submit Quiz 6 of 24 questions saved Page 6: op 2.
New tab /gastate.view.usg.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame auto.d21?ou=D1995113&isprv=&drc%3D0&qi=2970873&cfql=D0&dnb Final Exam (1151Lab) Time Limit: 1:00:00 Time Left:0:48:39 Sierra Reynolds: Attempt 1 Page 1: Previous Page Next Page Page 8 of 24 Question 8 (1 point) When magnesium metal is placed into hydrochloric acid, vigorous bubbling is observed. Which of the following is the Page 2: equation for this reaction? Remember, your answer must use correct chemical formulas of the reactants and products. Mg + 2HCI MgCl, + H, Mg + H2CI H2 + MgCl Page 3: Mg + Cl2 > MgCl2 Mg + HCI -> MgCl Page 4: Page 8 of 24 Previous Page Next Page Page 5; Submit Quiz 6 of 24 questions saved Page 6: op 2.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.17QAP
Related questions
Question
Help
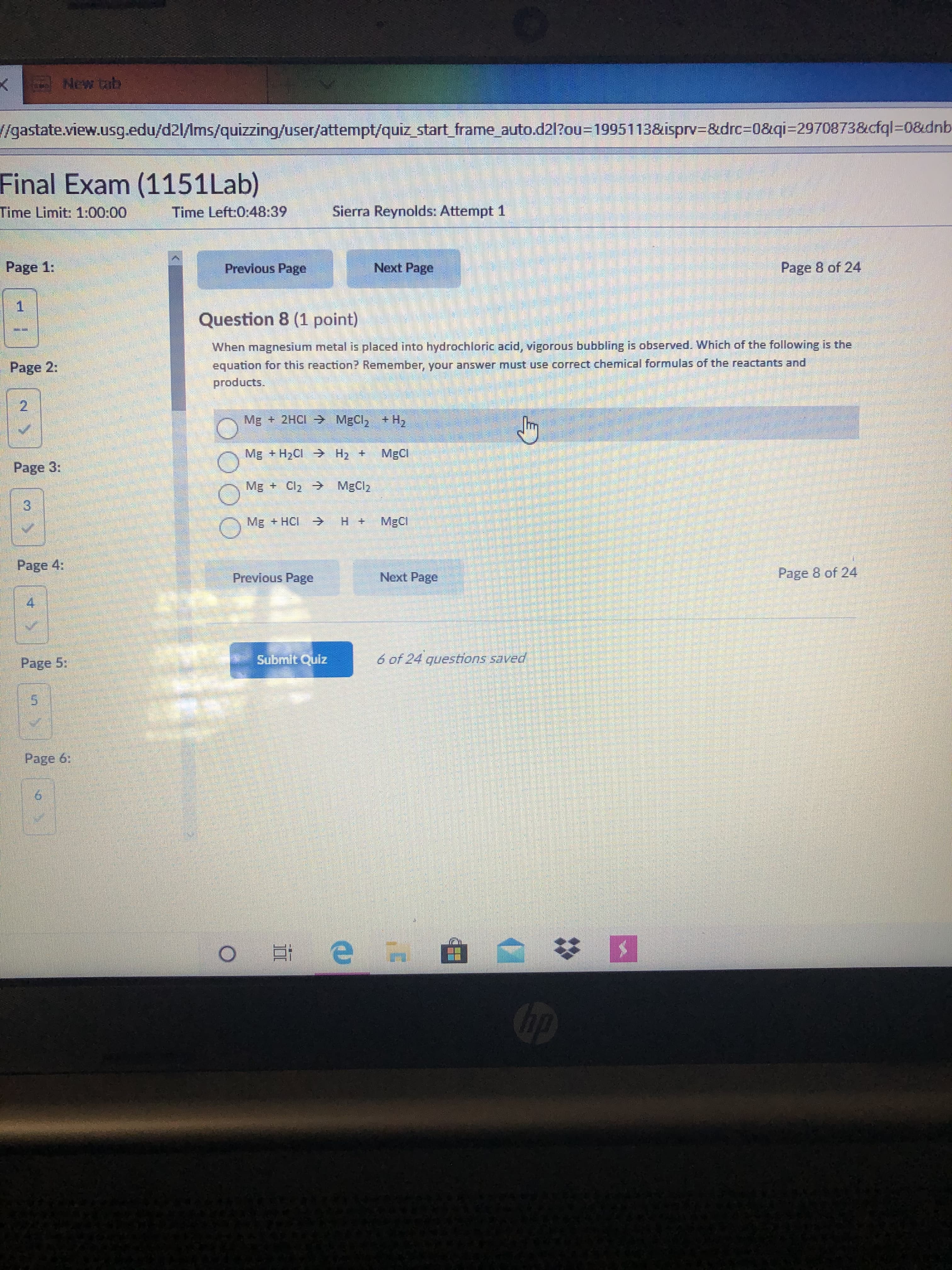
Transcribed Image Text:New tab
/gastate.view.usg.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame auto.d21?ou=D1995113&isprv=&drc%3D0&qi=2970873&cfql=D0&dnb
Final Exam (1151Lab)
Time Limit: 1:00:00
Time Left:0:48:39
Sierra Reynolds: Attempt 1
Page 1:
Previous Page
Next Page
Page 8 of 24
Question 8 (1 point)
When magnesium metal is placed into hydrochloric acid, vigorous bubbling is observed. Which of the following is the
Page 2:
equation for this reaction? Remember, your answer must use correct chemical formulas of the reactants and
products.
Mg + 2HCI MgCl, + H,
Mg + H2CI H2 +
MgCl
Page 3:
Mg + Cl2 > MgCl2
Mg + HCI ->
MgCl
Page 4:
Page 8 of 24
Previous Page
Next Page
Page 5;
Submit Quiz
6 of 24 questions saved
Page 6:
op
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

