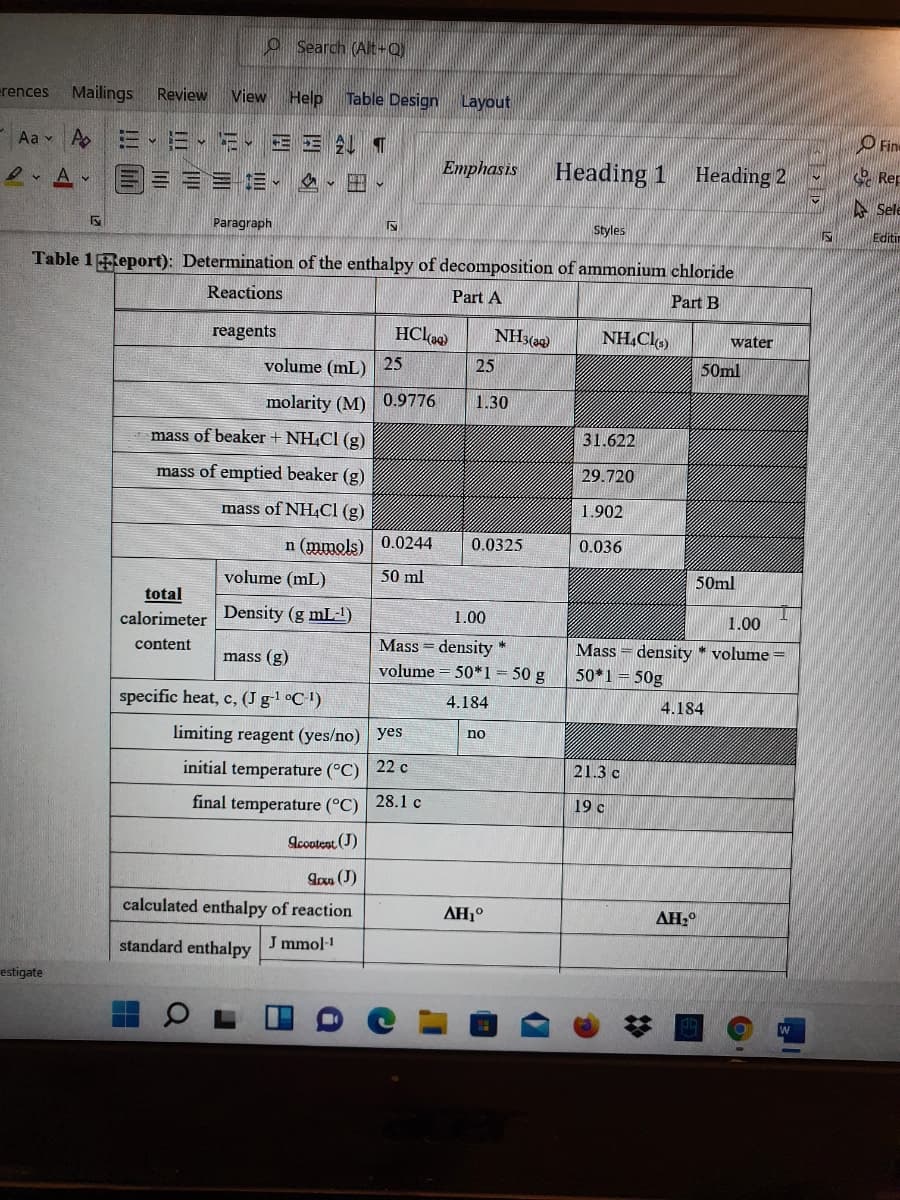
From the given information in the table, calculate: 1)qcontent(J) for part A and B 2) qrxn (J) for part A and B 3) standard enthalpy for part A and B 4) enthalpy of decomposition of NH4CL 5) literature enthalpy of decomposition of NH4Cl
From the given information in the table, calculate: 1)qcontent(J) for part A and B 2) qrxn (J) for part A and B 3) standard enthalpy for part A and B 4) enthalpy of decomposition of NH4CL 5) literature enthalpy of decomposition of NH4Cl
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 1.2ACP
Related questions
Question
From the given information in the table, calculate:
1)qcontent(J) for part A and B
2) qrxn (J) for part A and B
3) standard enthalpy for part A and B
4) enthalpy of decomposition of NH4CL
5) literature enthalpy of decomposition of NH4Cl
Transcribed Image Text:O Search (Alt+Q)
erences
Mailings
Review
View Help
Table Design
Layout
Aa v A
EE AL T
O Fin
Emphasis
Heading 1 Heading 2
三。
E Rep
A Sle
Paragraph
Styles
Editi
Table 1Report): Determination of the enthalpy of decomposition of ammonium chloride
Reactions
Part A
Part B
reagents
HCl
NH3(a9)
NH,Cl)
water
volume (mL) 25
25
50ml
molarity (M) 0.9776
1.30
mass of beaker + NH,CI (g)
31.622
mass of emptied beaker (g)
29.720
mass of NH,CI (g)
1.902
n (mmols) 0.0244
0.0325
0.036
volume (mL)
50 ml
50ml
total
calorimeter
Density (g mL1)
1.00
1.00
Mass = density *
volume = 50*1 = 50 g
content
Mass = density * volume =
50*1= 50g
mass (g)
specific heat, c, (J gl °C!)
4.184
4.184
limiting reagent (yes/no) yes
no
initial temperature (°C) 22 c
21.3 c
final temperature (°C) 28.1 c
19 c
Acootent (J)
Ion (J)
calculated enthalpy of reaction
AH1°
AH,
standard enthalpy
J mmol-1
estigate
Transcribed Image Text:O Search (Alt+Q)
ces
Mailings Review
View
Help
Table Design Layout
O Find -
a v
Emphasis
Heading 1
Heading 2
E Replace
A.
三。
A Select
Paragraph
Styles
Editing
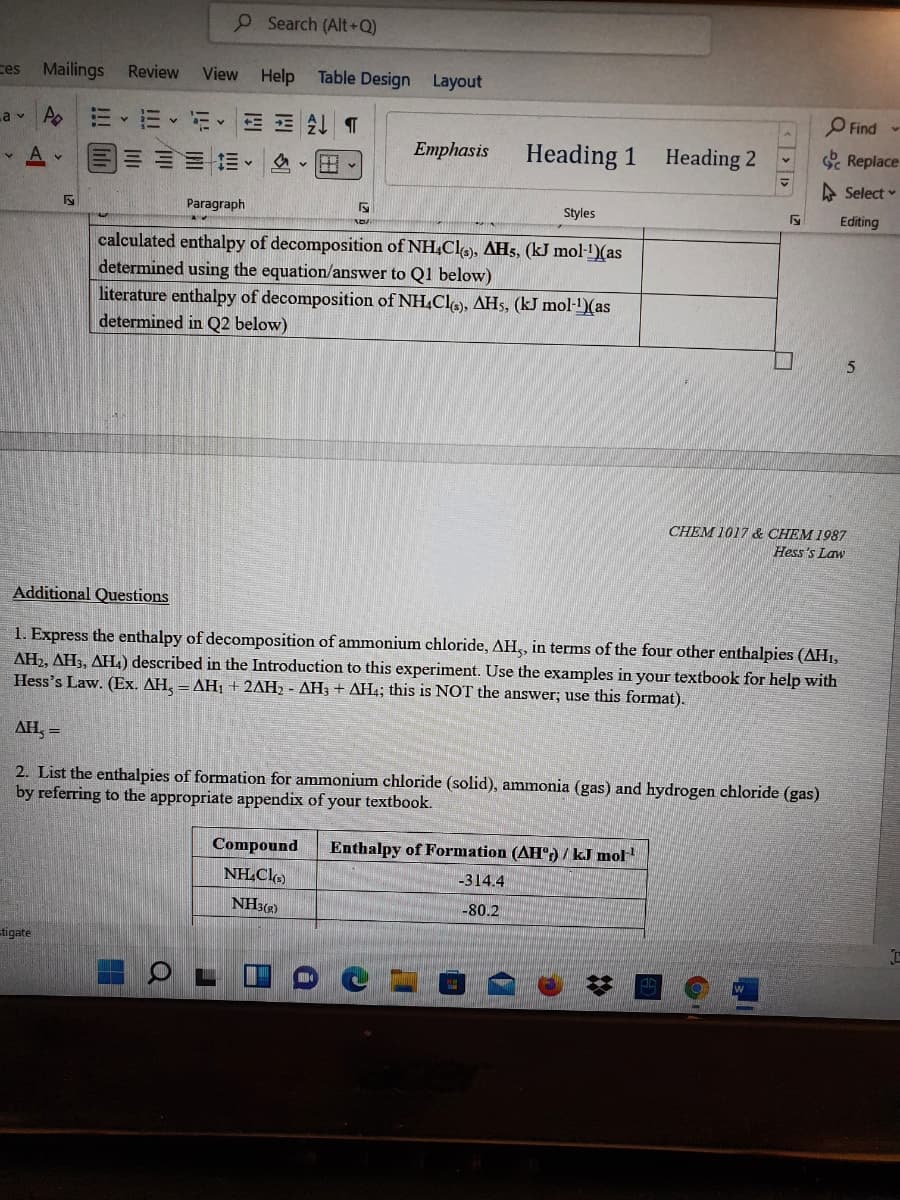
calculated enthalpy of decomposition of NH.Cle), AH5, (kJ mol-)(as
determined using the equation/answer to Q1 below)
literature enthalpy of decomposition of NH.Clo), AHs, (kJ mol-1)(as
determined in Q2 below)
CHEM 1017 & CHEM 1987
Hess's Law
Additional Questions
1. Express the enthalpy of decomposition of ammonium chloride, AH,, in terms of the four other enthalpies (AH1,
AH2, AH3, AH4) described in the Introduction to this experiment. Use the examples in your textbook for help with
Hess's Law. (Ex. AH, = AHỊ + 2AH2 - AH3 + AH4; this is NOT the answer; use this format).
AH, =
2. List the enthalpies of formation for ammonium chloride (solid), ammonia (gas) and hydrogen chloride (gas)
by referring to the appropriate appendix of your textbook.
Compound
Enthalpy of Formation (AH:) / kJ mol
NH.Cl
-314.4
NH3(2)
-80.2
tigate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning