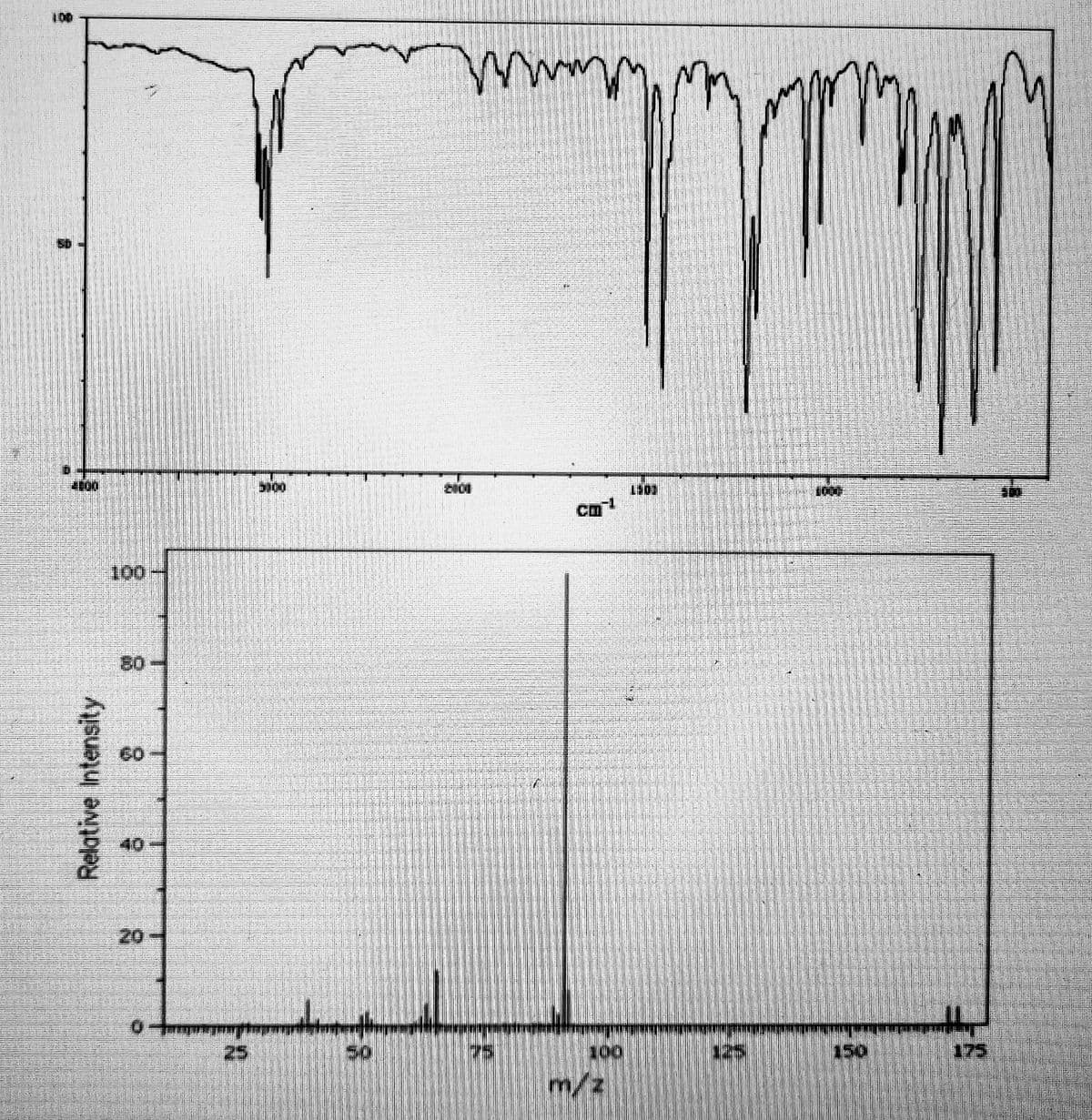
From the given IR and mass spectra of the unknown compound: 1. Which element is surely present in the compound? a. F b. O c. Br d. N 2. Which statement is true? a. It has no saturation b. It is an acid anhydride c. It contains -CH2- next to benzene ring d. All are false
From the given IR and mass spectra of the unknown compound: 1. Which element is surely present in the compound? a. F b. O c. Br d. N 2. Which statement is true? a. It has no saturation b. It is an acid anhydride c. It contains -CH2- next to benzene ring d. All are false
Chapter6: Random Errors In Chemical Analysis
Section: Chapter Questions
Problem 6.13QAP
Related questions
Question
From the given IR and mass spectra of the unknown compound:
1. Which element is surely present in the compound?
a. F
b. O
c. Br
d. N
2. Which statement is true?
a. It has no saturation
b. It is an acid anhydride
c. It contains -CH2- next to benzene ring
d. All are false
Transcribed Image Text:100
Cm
100
80
40
20
25
125
150
m/z
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

