Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
this is mass spectrum.
a. Methylcyclohexane
b. 2-Methyl-pentene
c. 2-methtl-2-hexanol
d. ethyl isobutyl ether
which is each proper structure?
what is each peak mean ?
How molecular ion to transfer eacg fragment?
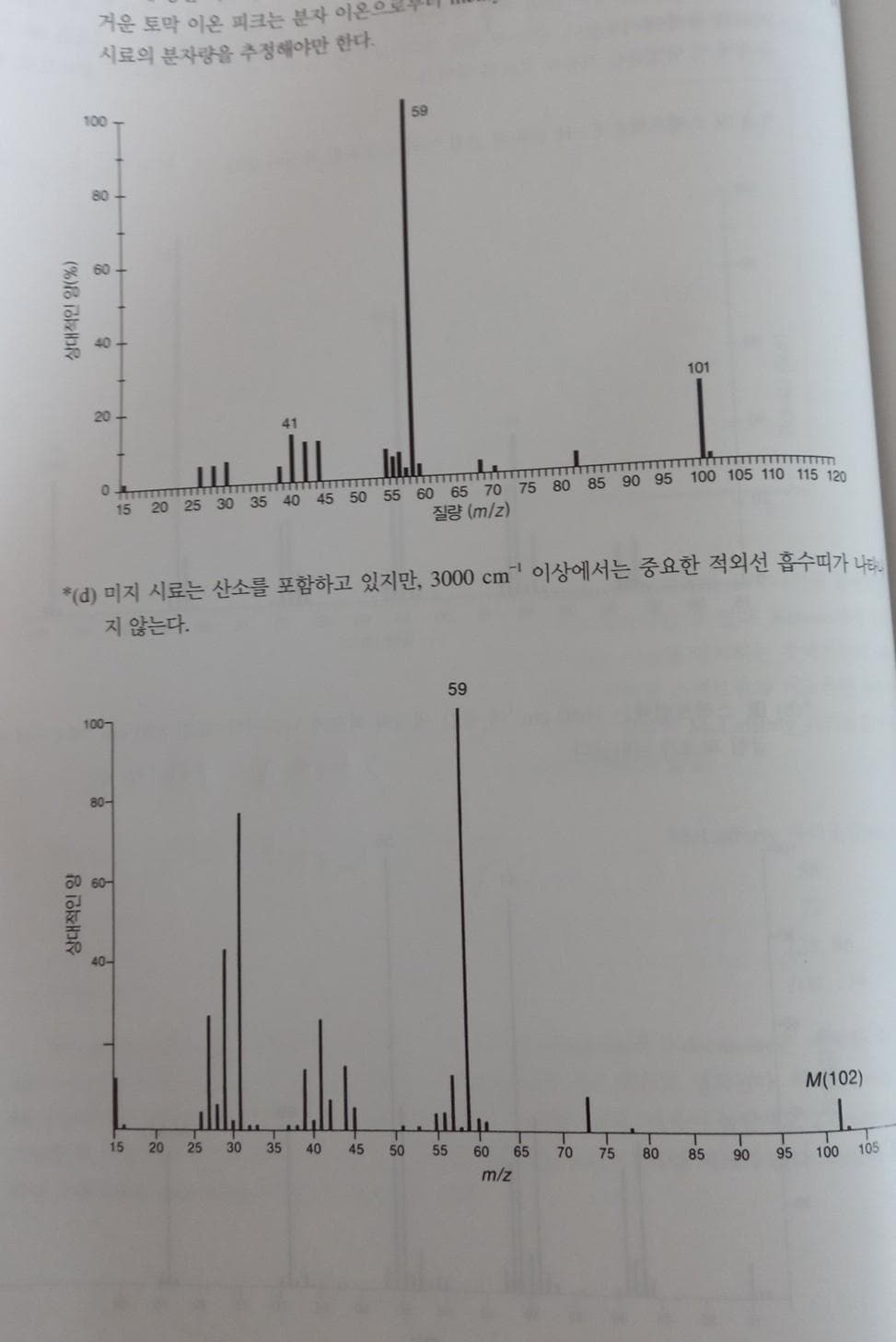
Transcribed Image Text:저운 토막 이온 피크는 분자 이온으
시료의 분자량을 추정해야만 한다.
100
59
80-
101
20
41
15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 105 110 115 120
질량 (m/z)
*(d) 미지 시료는 산소를 포함하고 있지만, 3000 cm" 이상에서는 중요한 적외선 흡수띠가 나타
지 않는다.
59
100-
80-
잉 60-
40-
M(102)
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
100
105
95
m/z
상대적인 양
상대적인 양(%)
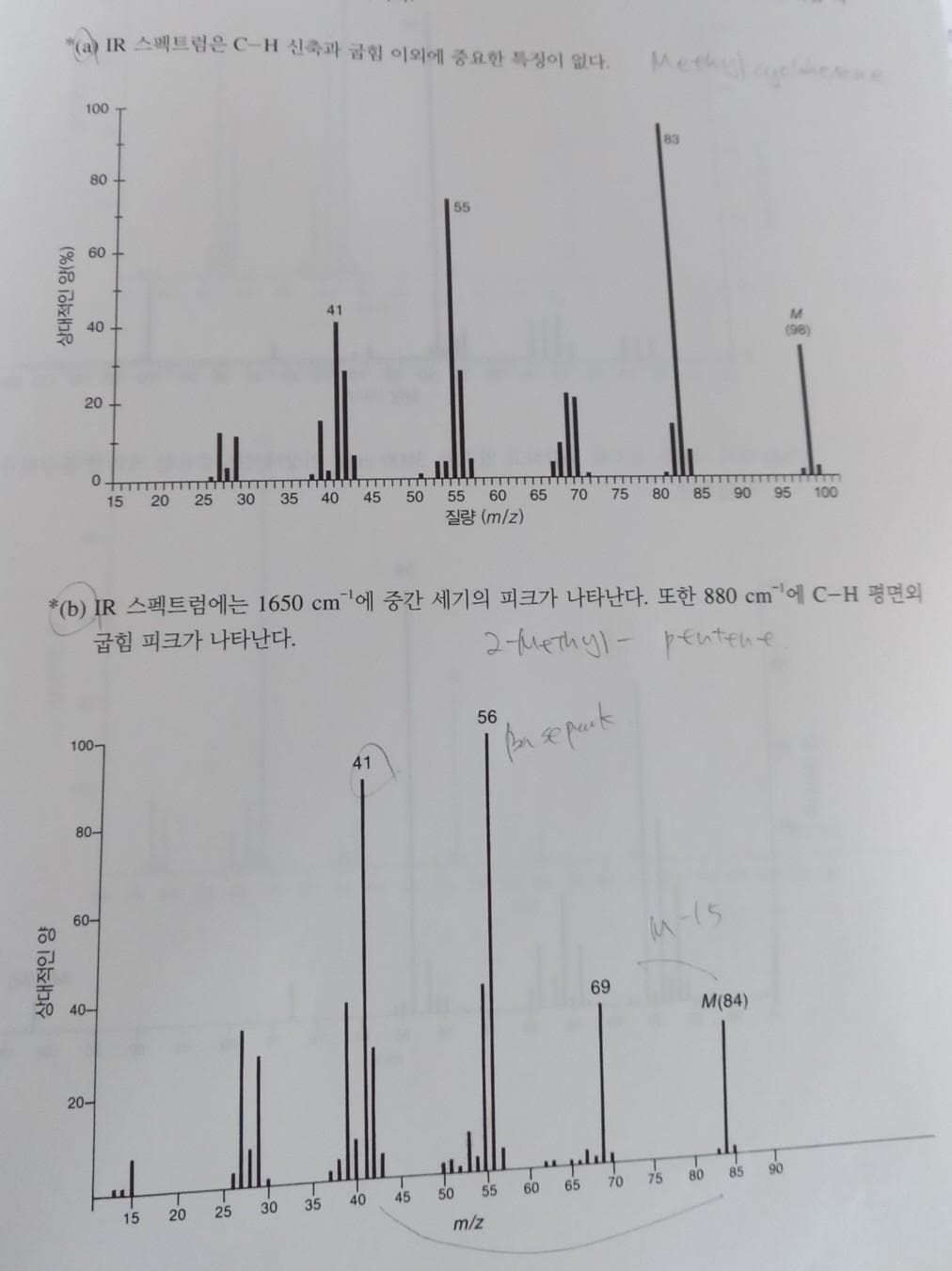
Transcribed Image Text:IR 스펙트럼은 C-H 신축과 금힘 이외에 중요한 특징이 없다.
Methulaeee
100
83
80
55
60
41
40 +
M.
(98)
20
TTTm T TTTTTT
15
20
25
30
35
40
45
50
55
60
65 70
75
80
85 90
95
100
질량 (m/z)
*(b) IR 스펙트럼에는 1650 cm'에 중간 세기의 피크가 나타난다. 또한 880 cm'에 C-H 평면의
굽힘 피크가 나타난다.
2-tuethyl- pentene
56
100-
41
80-
60-
40-
69
M(84)
20-
uplaly
70
75
80
85
90
35
40
45
50
55
60
65
15
20
25
30
m/z
상대적인 양
상대적인 양(%)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY