From the table of reagents shown below, show how you synthesize the product from the given reactant. Br Reagents available a. NBS, (PhCO2)2 h. CH3 Cl, AlCl3 CUCN 0. b. Br2, FeBr3 i. CH3 CH2Br p. H3O", A c. Br2, H+ j. CH3O¯, A q. CH3 CH2COCI, AICI3 r. H2NNH2, OH,A d. OH k. CH3 COCI, AIC13 e. NaOH(s), A 1. Mg, Et20 s. H2, Pа/C f. HNO3, H2 SO4 m. CO2(s) then H3O* t. H3PO2 g. H2 CrO4 n. HONO 0°C u. Cl2, FeCl3 (Enter the letter(s) of the reagent(s) needed in the box, in the order that they must be used. No more than two steps are required to perform the synthesis. Note that a retrosynthetic arrow is used, and therefore the reactant is shown on the right.) Answer:
From the table of reagents shown below, show how you synthesize the product from the given reactant. Br Reagents available a. NBS, (PhCO2)2 h. CH3 Cl, AlCl3 CUCN 0. b. Br2, FeBr3 i. CH3 CH2Br p. H3O", A c. Br2, H+ j. CH3O¯, A q. CH3 CH2COCI, AICI3 r. H2NNH2, OH,A d. OH k. CH3 COCI, AIC13 e. NaOH(s), A 1. Mg, Et20 s. H2, Pа/C f. HNO3, H2 SO4 m. CO2(s) then H3O* t. H3PO2 g. H2 CrO4 n. HONO 0°C u. Cl2, FeCl3 (Enter the letter(s) of the reagent(s) needed in the box, in the order that they must be used. No more than two steps are required to perform the synthesis. Note that a retrosynthetic arrow is used, and therefore the reactant is shown on the right.) Answer:
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
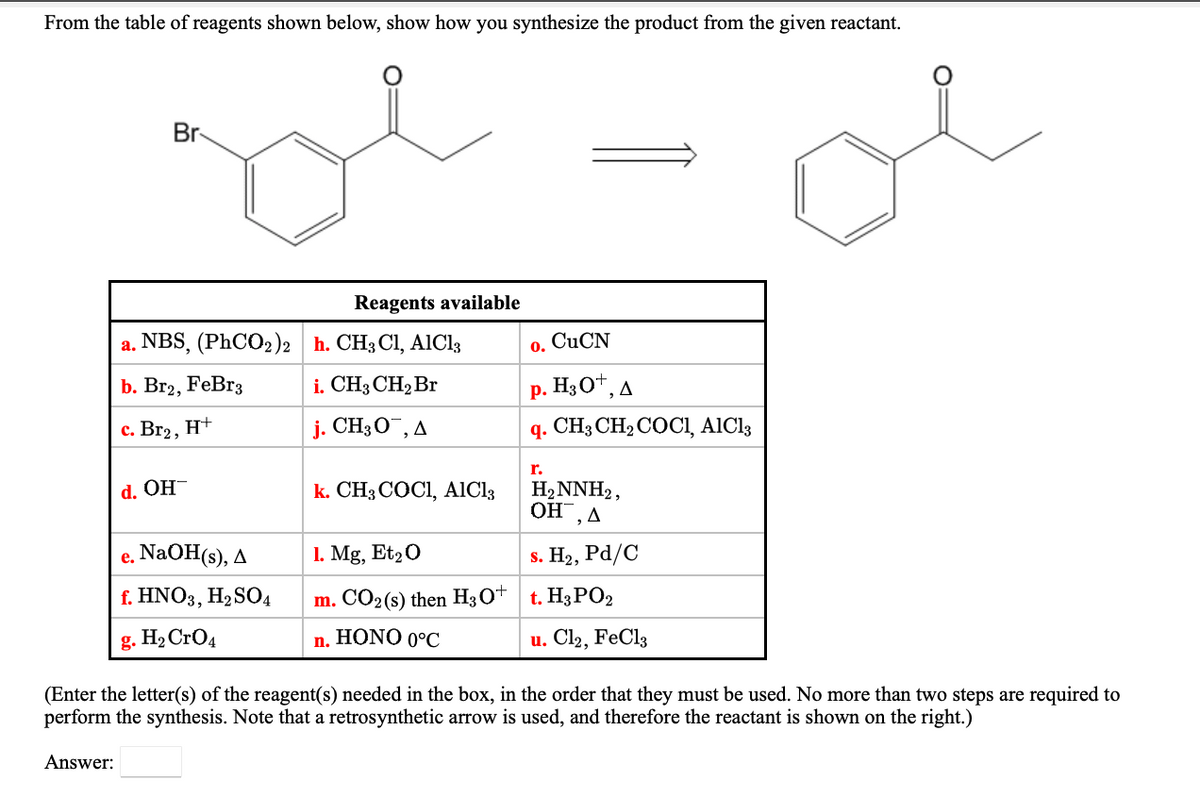
Transcribed Image Text:From the table of reagents shown below, show how you synthesize the product from the given reactant.
Br
Reagents available
a. NBS, (PhCO2)2 h. CH3 Cl, AIC13
CUCN
0.
b. Br2, FeBr3
i. CH3 CH2 Br
р. НзО*, д
с. Вг2, Н+
j. CH3O¯, A
q. CH; CH2 COCI, AICI3
r.
k. CH3 COCI, AICI3
H2NNH2,
OH, A
d. OH-
e. NaOH(s), A
1. Mg, Et20
s. H2, Pd/C
f. HNO3, H,SO4
m. CO2(s) then H3O+ t. H3PO2
g. H2 CrO4
n. HONO 0°C
u. Cl2, FeCl3
(Enter the letter(s) of the reagent(s) needed in the box, in the order that they must be used. No more than two steps are required to
perform the synthesis. Note that a retrosynthetic arrow is used, and therefore the reactant is shown on the right.)
Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning