Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 126CP: You have two distinct gaseous compounds made from element X and element Y. The mass percents are as...
Related questions
Question
From this data, what empirical formula of magnesium oxide.
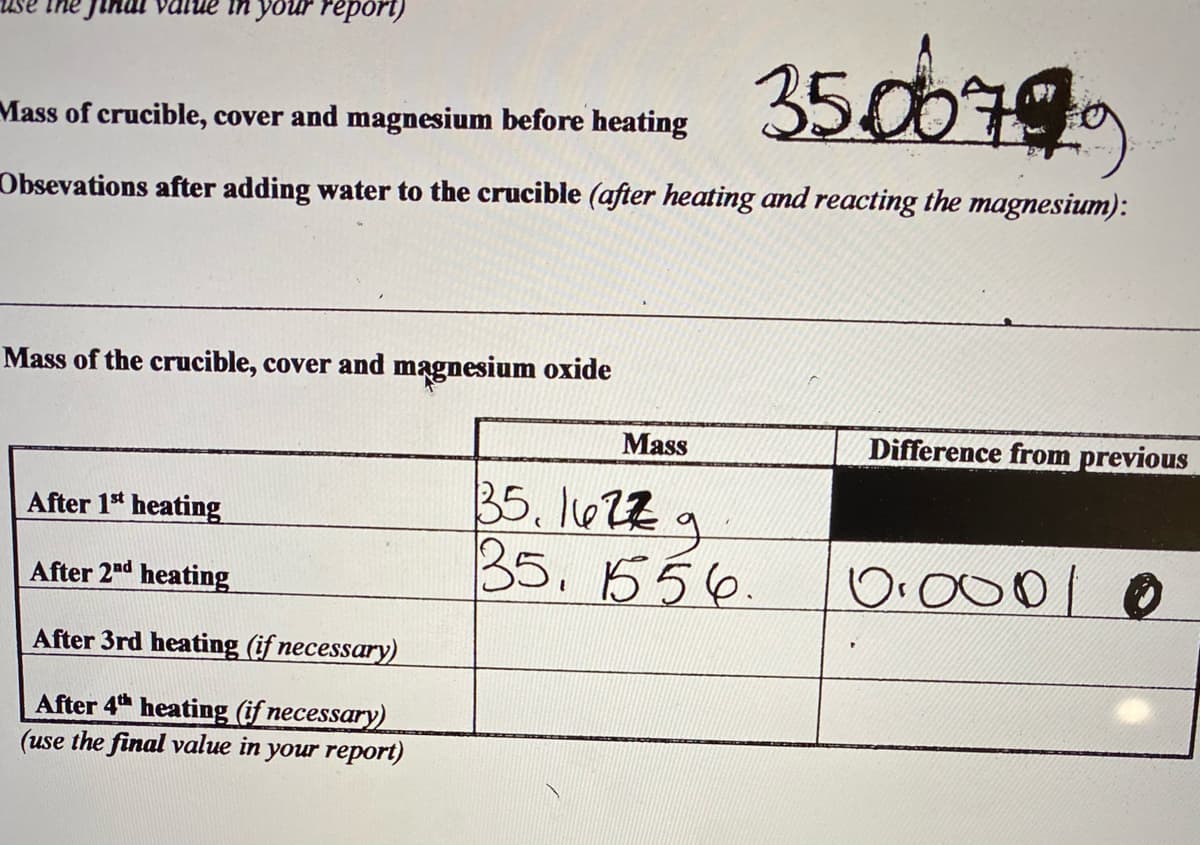
Transcribed Image Text:in your repori)
350079
Mass of crucible, cover and magnesium before heating
Obsevations after adding water to the crucible (after heating and reacting the magnesium):
Mass of the crucible, cover and magnesium oxide
Mass
Difference from previous
35. l022g
35.556.
After 1* heating
O.000 0
After 2nd heating
After 3rd heating (if necessary)
After 4 heating (if necessary)
(use the final value in your report)
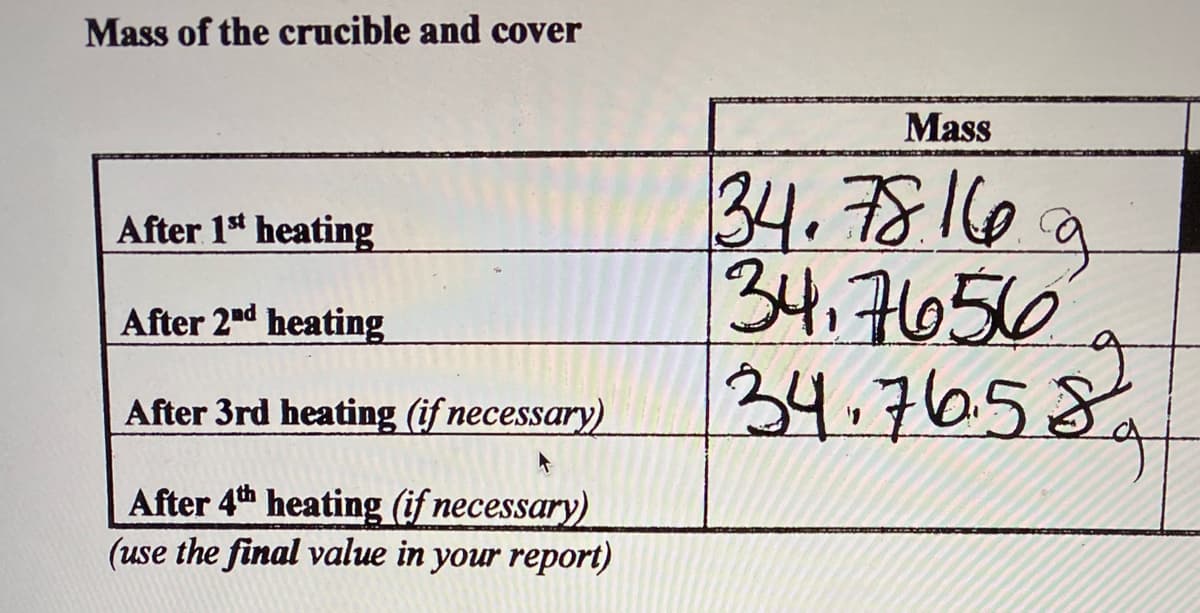
Transcribed Image Text:Mass of the crucible and cover
Mass
34.78116a
34,귀050
34.76.58.
After 1 heating
After 2nd heating
After 3rd heating (if necessary)
After 4th heating (if necessary)
(use the final value in your report)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning