(g) A different element, L, is located in the same row of the periodic table as element E, but L has roughly half the atomic mass of E. Is the atomic radius of L less than, equal to, or greater than the atomic radius of E? Justify your answer using principles of atomic structure. I x? x, 5 Ω 0 / 10000 Word Limit (h) The ionic radii of elements E and a different metallic element, M, are shown in the following table. Both elements form oxides, E20 and MO. If lattice energy is defined as the energy required to separate an ionic solid into individual separate gaseous ions, would the lattice energy of MO be less than, equal to, or greater than the lattice energy of the oxide E2O? Justify your answer in terms of Coulomb's law.
(g) A different element, L, is located in the same row of the periodic table as element E, but L has roughly half the atomic mass of E. Is the atomic radius of L less than, equal to, or greater than the atomic radius of E? Justify your answer using principles of atomic structure. I x? x, 5 Ω 0 / 10000 Word Limit (h) The ionic radii of elements E and a different metallic element, M, are shown in the following table. Both elements form oxides, E20 and MO. If lattice energy is defined as the energy required to separate an ionic solid into individual separate gaseous ions, would the lattice energy of MO be less than, equal to, or greater than the lattice energy of the oxide E2O? Justify your answer in terms of Coulomb's law.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter8: Electron Configurations And Periodicity
Section: Chapter Questions
Problem 8.37QP: Two elements are in the same group, one following the other. One is a metalloid; the other is a...
Related questions
Question
I need help understanding these questions
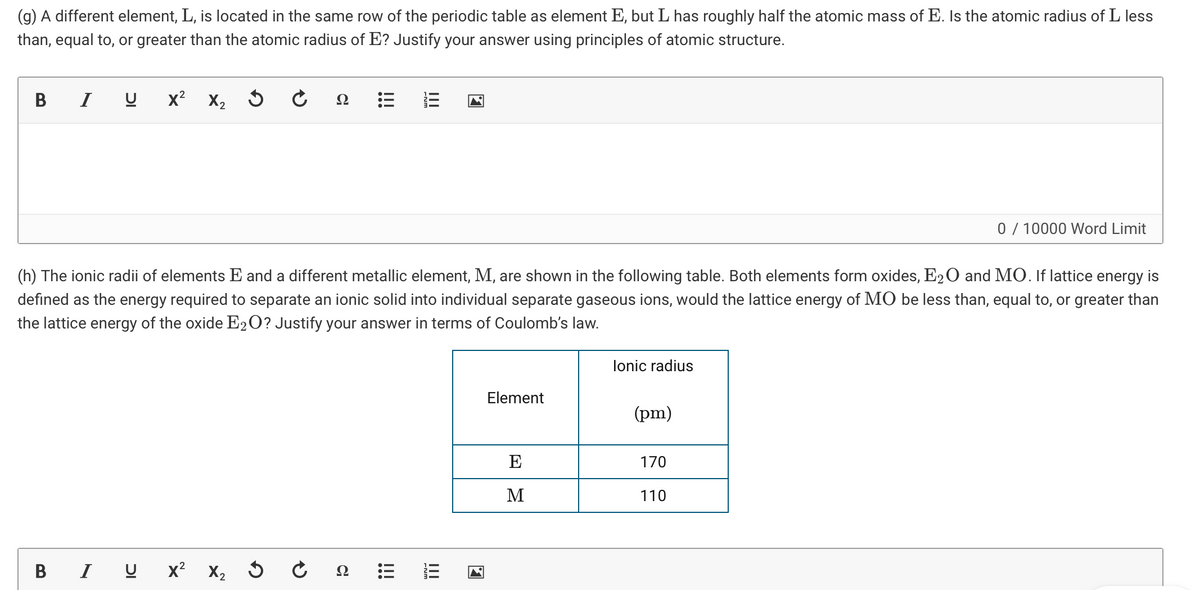
Transcribed Image Text:(g) A different element, L, is located in the same row of the periodic table as element E, but L has roughly half the atomic mass of E. Is the atomic radius of L less
than, equal to, or greater than the atomic radius of E? Justify your answer using principles of atomic structure.
В
I
x2 X2
Ω
0 / 10000 Word Limit
(h) The ionic radii of elements E and a different metallic element, M, are shown in the following table. Both elements form oxides, E20 and MO. If lattice energy is
defined as the energy required to separate an ionic solid into individual separate gaseous ions, would the lattice energy of MO be less than, equal to, or greater than
the lattice energy of the oxide E2O? Justify your answer in terms of Coulomb's law.
lonic radius
Element
(pm)
E
170
M
110
B I U x? X2
Ω
!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: please give me answer in relatable
Q: Questions 3~5 are a set of questions. Here is the table for the questions.
Output Total Cost
0
$0
1…
Q: 2.
I mix 1 L of 0.1 M ammonium phosphate with 1 L of 0.1 M stronti
of reaction will occur?
a. Redox…
Q: JLK/m
23. Nitric oxide reacts with chlorine to form NOCI. 1.
2NO(g) + Cl2(g) → 2NOCI(g)
Substance:…
Q: please answer in text form and in proper format answer with must explanation , calculation for each…
Q: 11
Logistic Regression Output – Example 2
A credit card company wants to predict if an existing card…
Q: 1. Which of the following statements are correct? (i) When average variable cost
is rising, marginal…
Q: Use the standard reduction potentials located in the 'Tables' linked above to calculate the…
Q: please answer in text form and in proper format answer with must explanation , calculation for each…
Q: The price of Ervin Corp. stock will either be $63 or $82 at the end of the
year. Call options are…
Q: please answer in text form and in proper format answer with must explanation , calculation for each…
Q: Project L requires an initial outlay at t 0 of $46,000, its expected cash inflows are $13,000 per…
Q: The graph demonstrates the domestic demand and supply for a good, as well as the world price for…
Q: QUESTION 18
What are the best reagents to complete this transformation?
OH
OHg(OAc)2/H2O followed by…
Q: PQ-17. A sample of ethanol (C2H6O) contains 3.024 g of hydrogen. How many moles of carbon are in the…
Q: Write the structure of all organic products formed in the following reaction.
NaBH4
Q: all lone-pair electrons and unpaired electrons. Hint: the radicals do not coexist in the same…
Q: A mortgage that allows the borrower to pay less than the interest due for a few year
(A)…
Q: please answer in text form and in proper format answer with must explanation , calculation for each…
Q: Determine (a) the x2 test statistic, (b) the degrees of freedom, (c) the
critical value using x =…
Q: Mary is a sales person for Challenge Furniture. She receives an incremental commission based on the…