Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 80QAP: In the photoelectric effect, electrons are ejected from a metal surface when light strikes it. A...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
Do question 4 please and make your handwriting clear
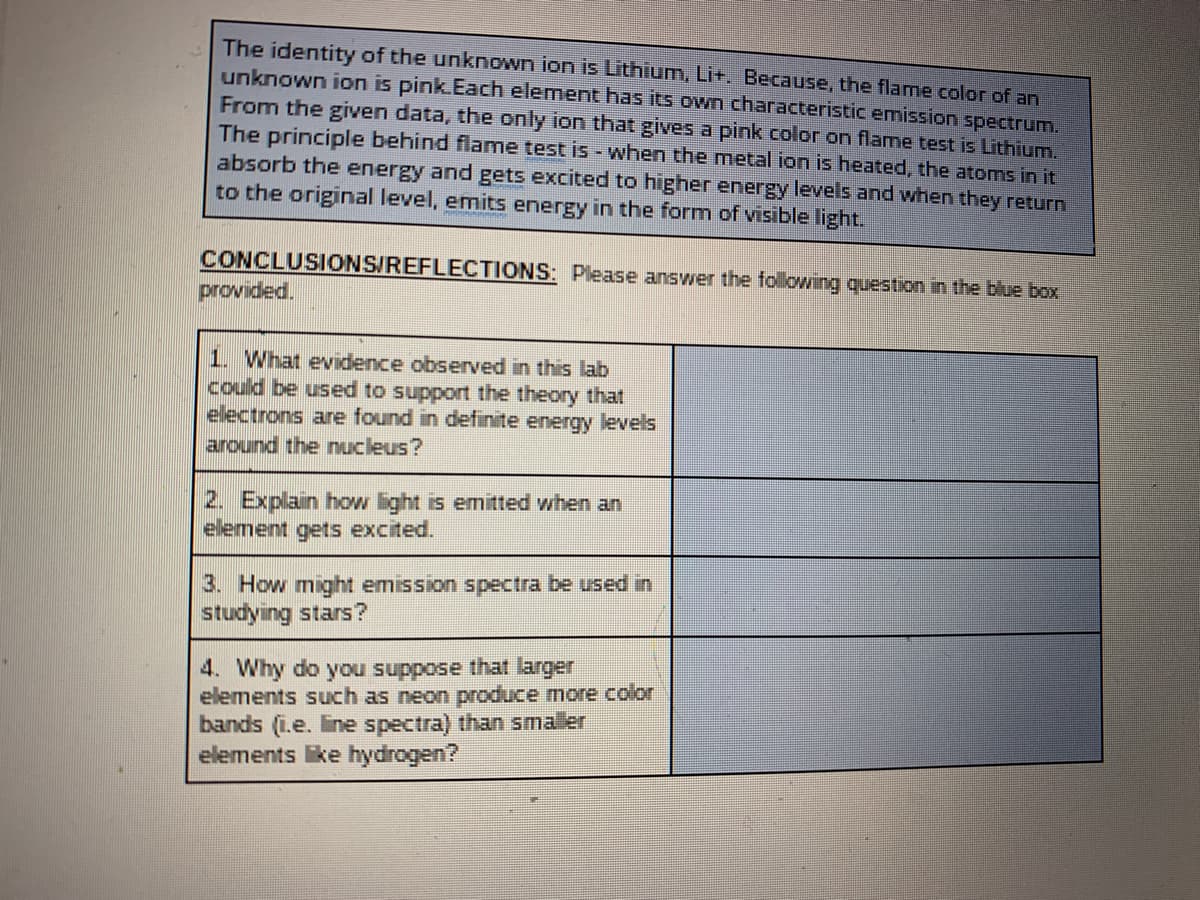
Transcribed Image Text:The identity of the unknown ion is Lithium, Lit. Because, the flame color of an
unknown ion is pink.Each element has its own characteristic emission spectrum.
From the given data, the only ion that gives a pink color on flame test is Lithium.
The principle behind flame test is - when the metal ion is heated, the atoms in it
absorb the energy and gets excited to higher energy levels and when they return
to the original level, emits energy in the form of visible light.
CONCLUSIONS/REFLECTIONS: Please answer the following questiion in the blue box
provided.
1. What evidence observed in this lab
could be used to support the theory that
electrons are found in definite energy levels
around the nucleus?
2. Explain how light is emitted when an
element gets excited.
3. How might emission spectra be used in
studying stars?
4. Why do you suppose that larger
elements such as neon produce more color
bands (i.e. line spectra) than smaller
elements ke hydrogen?
Transcribed Image Text:Accessibility
Last edit was yesterday at 9:07 PM
IUA
10.5
三 -=
2...
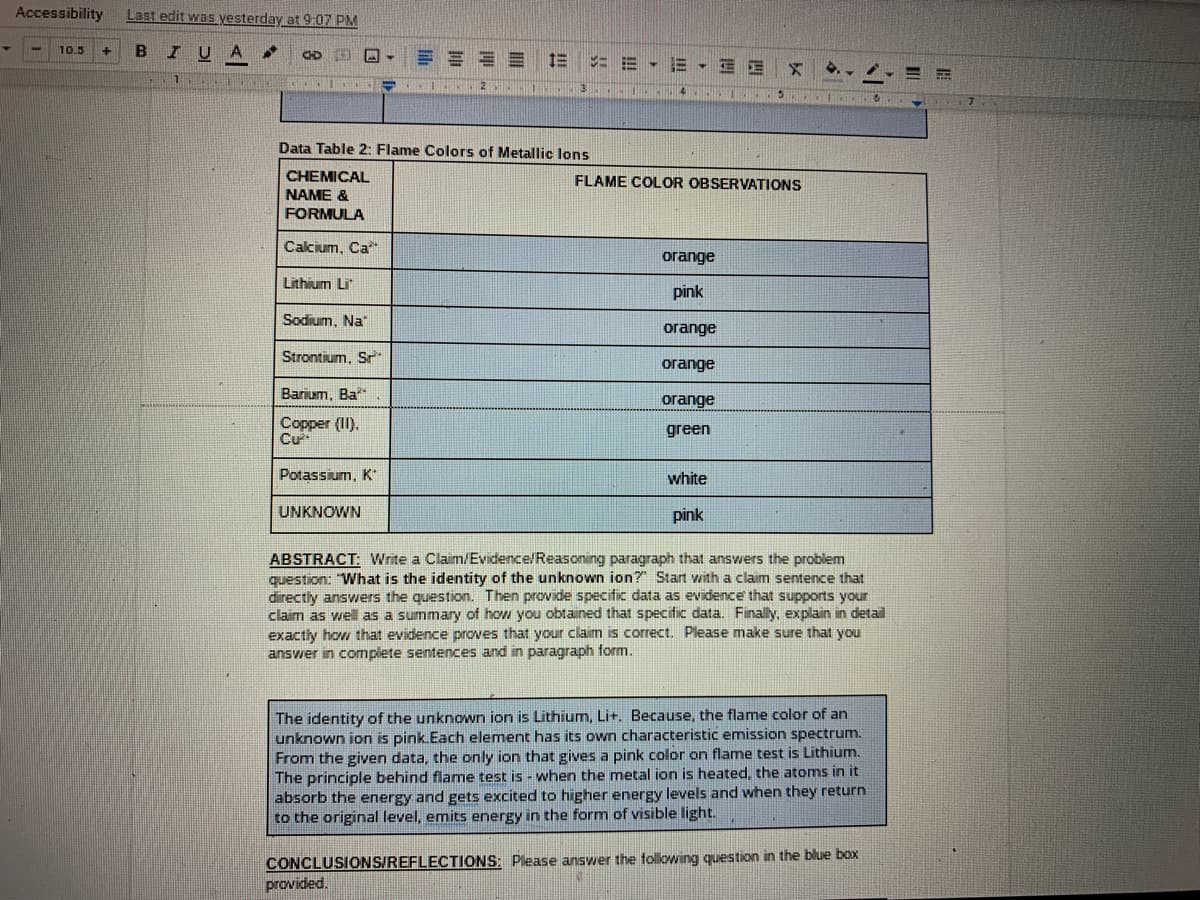
Data Table 2: Flame Colors of Metallic lons
CHEMICAL
NAME &
FLAME COLOR OBSERVATIONS
FORMULA
Calcium, Ca*
orange
Lithium Li
pink
Sodium, Na
orange
Strontium, Sr
огаnge
Barium, Ba
orange
Copper (II).
Cư
green
Potassium, K
white
UNKNOWN
pink
ABSTRACT: Write a Claim/Evidence/Reasoning paragraph that answers the problem
question: "What is the identity of the unknown ion?" Start with a claim sentence that
directly answers the question. Then provide specific data as evidence that supports your
claim as well as a summary of how you obtained that specific data. Finally, explain in detail
exactly how that evidence proves that your claim is correct. Please make sure that you
answer in complete sentences and in paragraph form.
The identity of the unknown ion is Lithium, Lit. Because, the flame color of an
unknown ion is pink.Each element has its own characteristic emission spectrum.
From the given data, the only ion that gives a pink color on flame test is Lithium.
The principle behind flame test is - when the metal ion is heated, the atoms in it
absorb the energy and gets excited to higher energy levels and when they return
to the original level, emits energy in the form of visible light.
CONCLUSIONS/REFLECTIONS: Please answer the following question in the blue boxx
provided.
四
III
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning