Gas chromatography can be used to evaluate thermodynamic parameters of volatile compounds such as those shown below. Calculate the heat of adsorption of the following alkanes on activated alumina based on the following Henry's Law Constants. Explain the trend in enthalpies and entropies of adsorption for the four alkanes. Henry's Law Constants for n-Alkane Adsorption on Activated Alumina Compound 185 °C 195 °C 205 °C 215 °C n-Pentane 1.292 1.034 0.863 0.719 n-Hexane 2.894 2.167 1.712 1.387 n-Heptane 7.297 5.564 4.414 3.184
Gas chromatography can be used to evaluate thermodynamic parameters of volatile compounds such as those shown below. Calculate the heat of adsorption of the following alkanes on activated alumina based on the following Henry's Law Constants. Explain the trend in enthalpies and entropies of adsorption for the four alkanes. Henry's Law Constants for n-Alkane Adsorption on Activated Alumina Compound 185 °C 195 °C 205 °C 215 °C n-Pentane 1.292 1.034 0.863 0.719 n-Hexane 2.894 2.167 1.712 1.387 n-Heptane 7.297 5.564 4.414 3.184
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section: Chapter Questions
Problem 20.25P
Related questions
Question
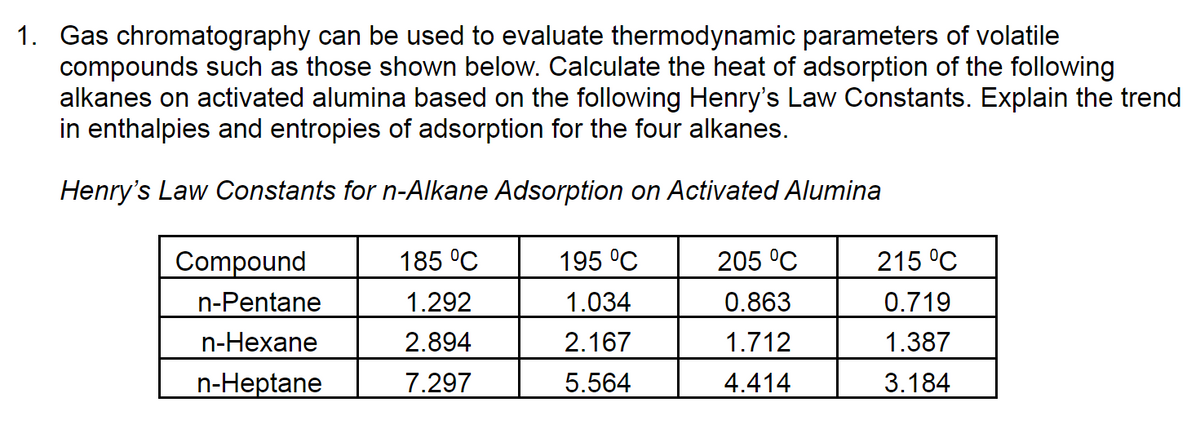
Transcribed Image Text:1. Gas chromatography can be used to evaluate thermodynamic parameters of volatile
compounds such as those shown below. Calculate the heat of adsorption of the following
alkanes on activated alumina based on the following Henry's Law Constants. Explain the trend
in enthalpies and entropies of adsorption for the four alkanes.
Henry's Law Constants for n-Alkane Adsorption on Activated Alumina
Compound
185 °C
195 °C
205 °C
215 °C
n-Pentane
1.292
1.034
0.863
0.719
n-Hexane
2.894
2.167
1.712
1.387
n-Heptane
7.297
5.564
4.414
3.184
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning