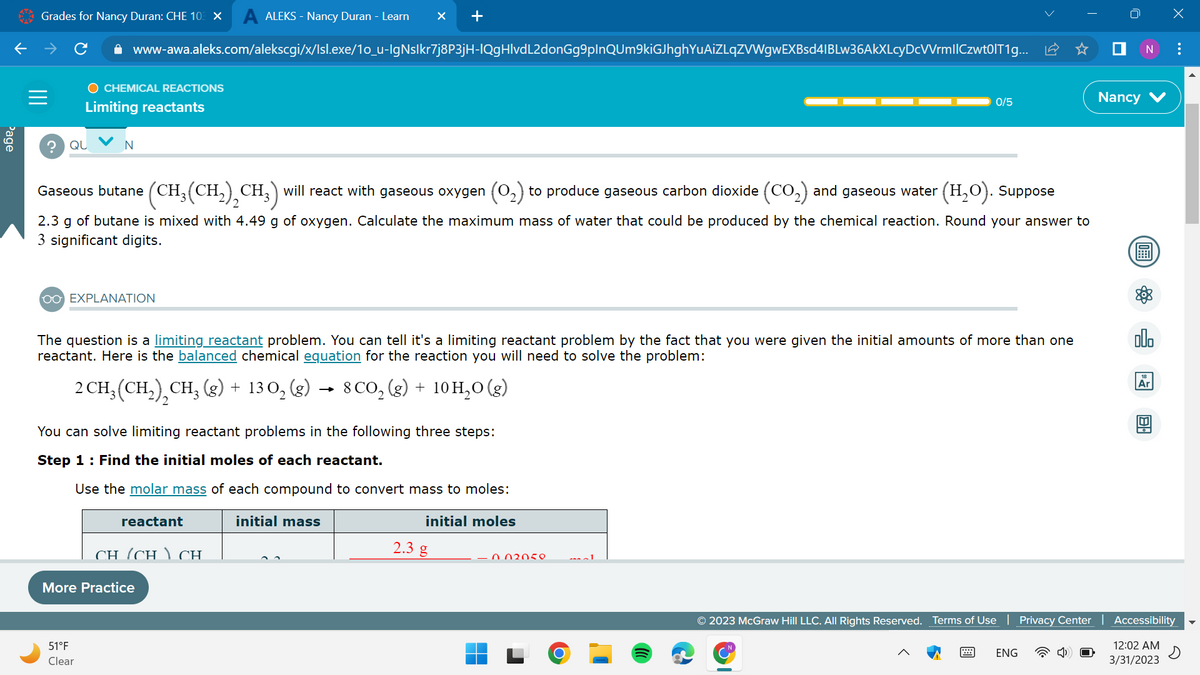
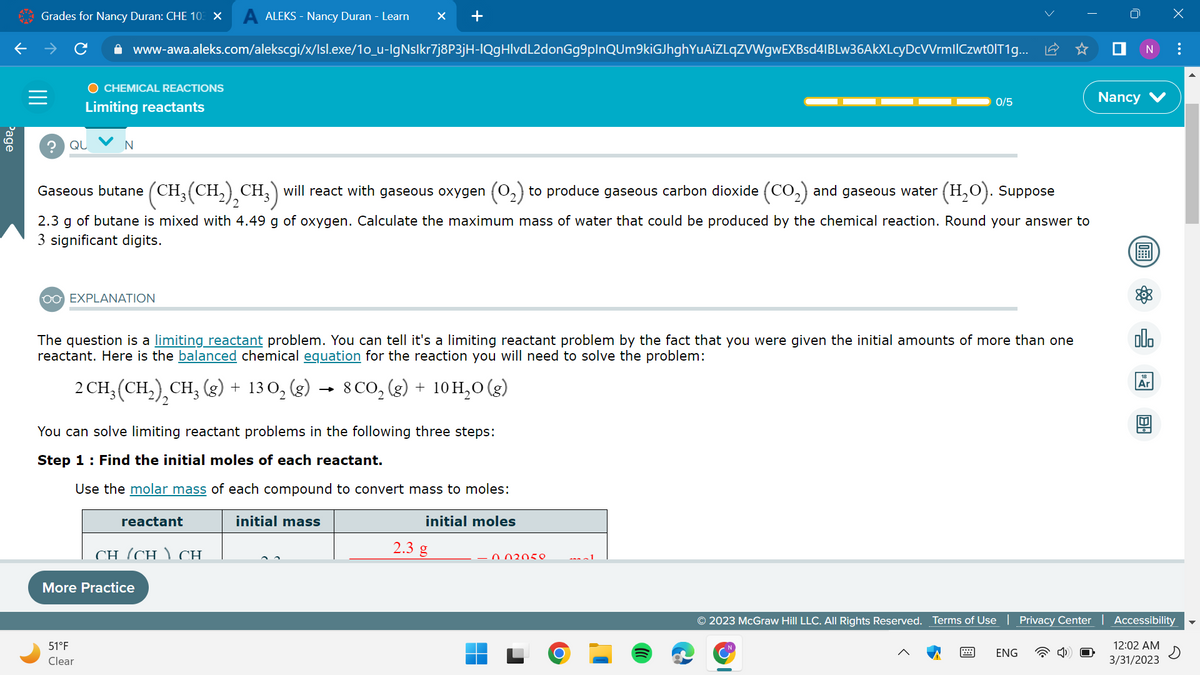
Gaseous butane (CH. (CH₂(CH₂)₂CH₂) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 2.3 g of butane is mixed with 4.49 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits.
Gaseous butane (CH. (CH₂(CH₂)₂CH₂) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 2.3 g of butane is mixed with 4.49 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 3 significant digits.
Chapter13: Kinetic Methods
Section: Chapter Questions
Problem 6P
Related questions
Question
Transcribed Image Text:Page
Grades for Nancy Duran: CHE 103 X A ALEKS - Nancy Duran - Learn × +
✰ www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQgHlvdL2donGg9plnQUm9kiGJhghYuAiZLqZVWgwEXBsd4IBLw36AkXLcyDcVVrmllCzwt0IT1g...
?
O CHEMICAL REACTIONS
Limiting reactants
QU
N
OO EXPLANATION
Gaseous butane (CH₂(CH₂)₂CH3) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water
(H₂O). Suppose
2.3 g of butane is mixed with 4.49 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to
3 significant digits.
The question is a limiting reactant problem. You can tell it's a limiting reactant problem by the fact that you were given the initial amounts of more than one
reactant. Here is the balanced chemical equation for the reaction you will need to solve the problem:
2 CH₂ (CH₂), CH₂(g) + 130₂ (g) → 8 CO₂(g) + 10 H₂O(g)
You can solve limiting reactant problems in the following three steps:
Step 1: Find the initial moles of each reactant.
Use the molar mass of each compound to convert mass to moles:
initial mass
initial moles
51°F
Clear
reactant
CH (CH) CH
More Practice
2.3 g
0/5
0.02050
1
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use
T
ENG
■ N :
Nancy
(OFF
olo
Ar
X
de
Privacy Center | Accessibility
12:02 AM
3/31/2023
Transcribed Image Text:Page
Grades for Nancy Duran: CHE 103 X A ALEKS - Nancy Duran - Learn × +
✰ www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQgHlvdL2donGg9plnQUm9kiGJhghYuAiZLqZVWgwEXBsd4IBLw36AkXLcyDcVVrmllCzwt0IT1g...
?
O CHEMICAL REACTIONS
Limiting reactants
QU
N
OO EXPLANATION
Gaseous butane (CH₂(CH₂)₂CH3) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water
(H₂O). Suppose
2.3 g of butane is mixed with 4.49 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to
3 significant digits.
The question is a limiting reactant problem. You can tell it's a limiting reactant problem by the fact that you were given the initial amounts of more than one
reactant. Here is the balanced chemical equation for the reaction you will need to solve the problem:
2 CH₂ (CH₂), CH₂(g) + 130₂ (g) → 8 CO₂(g) + 10 H₂O(g)
You can solve limiting reactant problems in the following three steps:
Step 1: Find the initial moles of each reactant.
Use the molar mass of each compound to convert mass to moles:
initial mass
initial moles
51°F
Clear
reactant
CH (CH) CH
More Practice
2.3 g
0/5
0.02050
1
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use
T
ENG
■ N :
Nancy
(OFF
olo
Ar
X
de
Privacy Center | Accessibility
12:02 AM
3/31/2023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning