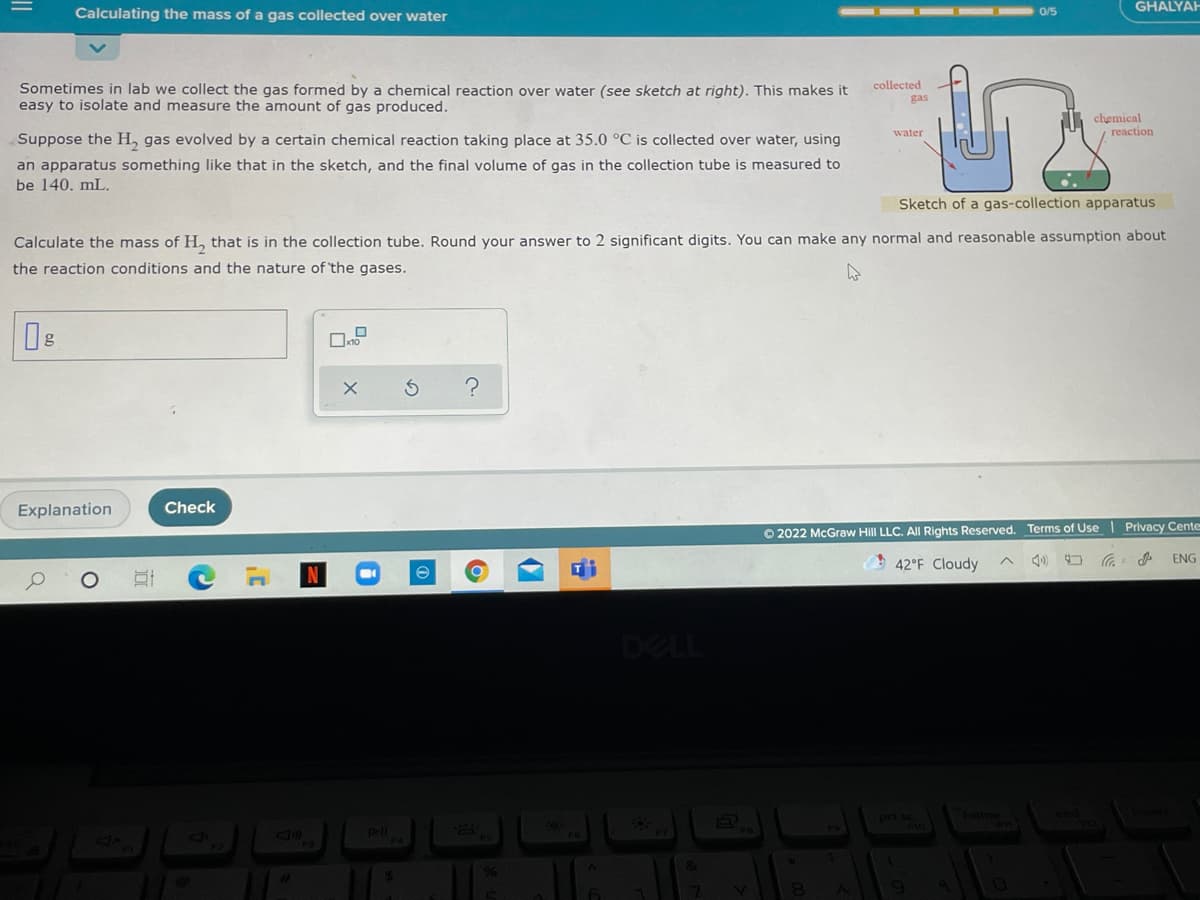
GHA Calculating the mass of a gas collected over water 0/5 Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. collected gas chemical reaction water Suppose the H, gas evolved by a certain chemical reaction taking place at 35.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 140. mL. Sketch of a gas-collection apparatus Calculate the mass of H, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases.
GHA Calculating the mass of a gas collected over water 0/5 Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. collected gas chemical reaction water Suppose the H, gas evolved by a certain chemical reaction taking place at 35.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 140. mL. Sketch of a gas-collection apparatus Calculate the mass of H, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.28QP
Related questions
Question
100%
Transcribed Image Text:GHALYAH
Calculating the mass of a gas collected over water
0/5
collected
Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it
easy to isolate and measure the amount of gas produced.
gas
chemical
reaction
water
Suppose the H, gas evolved by a certain chemical reaction taking place at 35.0 °C is collected over water, using
an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to
be 140. mL.
Sketch of a gas-collection apparatus
Calculate the mass of H, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about
the reaction conditions and the nature of 'the gases.
Explanation
Check
O 2022 McGraw Hill LLC. AlI Rights Reserved. Terms of Use | Privacy Cente
42°F Cloudy
ENG
DELL
3Sud
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,