Give a molecular formula for your product if it contains no oxygens. Give the molecular formulas if your product contains one and two oxygens (some may not be possible). The molecular formula for if my molecule does not have any oxygens is C6H6, if it has one oxygen it would be C7H6O, if it was two it would be C8H7O2. Calculate the index of hydrogen deficiency, and therefore the number of rings and/or p- bonds in your unknown for each of the molecular formulas in question 3. Show your calculation
Give a molecular formula for your product if it contains no oxygens. Give the molecular formulas if your product contains one and two oxygens (some may not be possible). The molecular formula for if my molecule does not have any oxygens is C6H6, if it has one oxygen it would be C7H6O, if it was two it would be C8H7O2. Calculate the index of hydrogen deficiency, and therefore the number of rings and/or p- bonds in your unknown for each of the molecular formulas in question 3. Show your calculation
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section20.3: Uv-visible Spectroscopy
Problem 20.5P
Related questions
Question
- Give a molecular formula for your product if it contains no oxygens. Give the molecular formulas if your product contains one and two oxygens (some may not be possible).
The molecular formula for if my molecule does not have any oxygens is C6H6, if it has one oxygen it would be C7H6O, if it was two it would be C8H7O2.
Calculate the index of hydrogen deficiency, and therefore the number of rings and/or p- bonds in your unknown for each of the molecular formulas in question 3. Show your
calculation
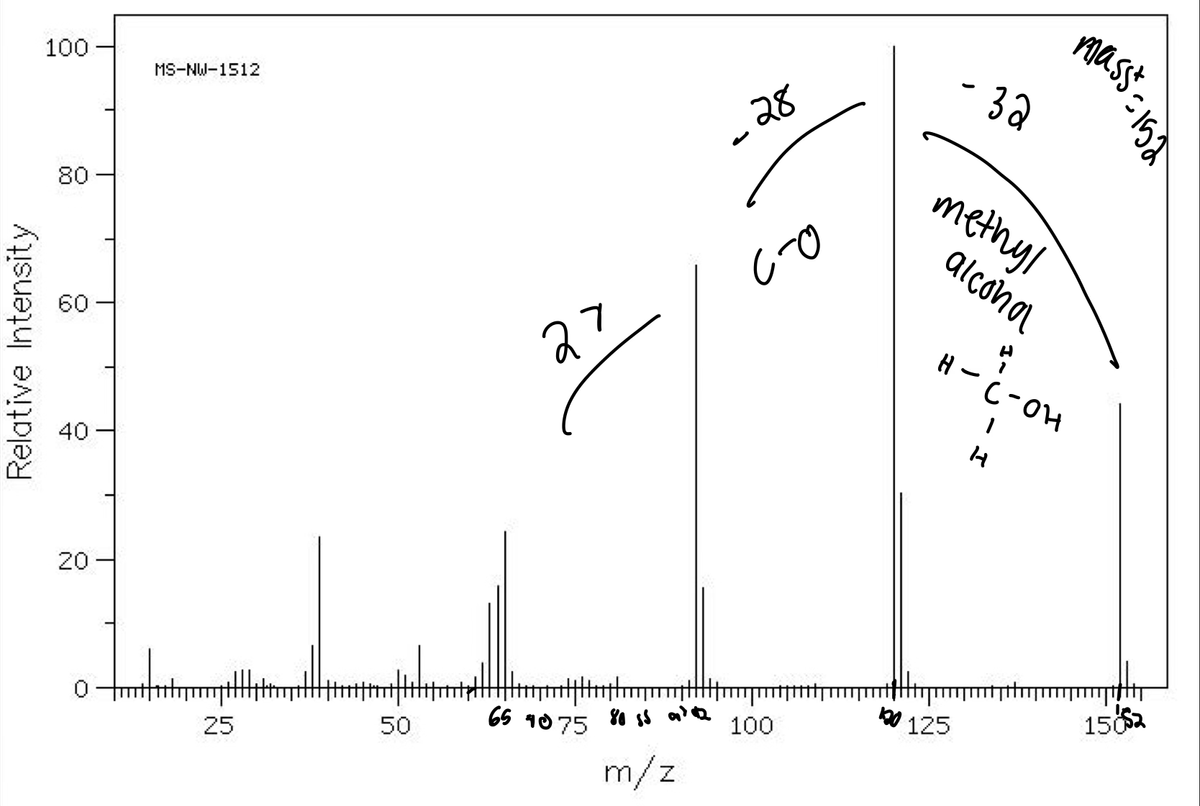
Transcribed Image Text:- 32
100
MS-NW-1512
„28
methyl
80
alconal
60
27
H- C-OH
40
20
b0 125
1552
65
1075
100
50
25
m/z
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning