Given a barometric pressure of 762.4 mmHg, calculate the pres- sure of each gas sample as indicated by the manometer. Atmospheric Atmospheric pressure pressure cm cm h Mercury Mercury (a) (b)
Given a barometric pressure of 762.4 mmHg, calculate the pres- sure of each gas sample as indicated by the manometer. Atmospheric Atmospheric pressure pressure cm cm h Mercury Mercury (a) (b)
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 82P: The energy released from condensation in thunderstorms can be very large. Calculate the energy...
Related questions
Question
100%
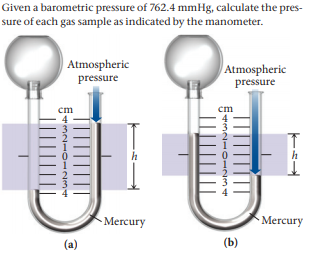
Transcribed Image Text:Given a barometric pressure of 762.4 mmHg, calculate the pres-
sure of each gas sample as indicated by the manometer.
Atmospheric
Atmospheric
pressure
pressure
cm
cm
h
Mercury
Mercury
(a)
(b)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

