Given: Fe * (a) t 2e teu). E0:-0.49V fe * E 0.04. (aq) +3e te la). Fe "( aqlte 7 Fe "(aq). Cl2(g) + 2e 7 201" (aq). Eo +0 77 V 31 E : +1-36 V Caloulate the AE° for the reaction 2t Fe(s) + Clacg) - te "(aq) t 2C1 (aq)
Given: Fe * (a) t 2e teu). E0:-0.49V fe * E 0.04. (aq) +3e te la). Fe "( aqlte 7 Fe "(aq). Cl2(g) + 2e 7 201" (aq). Eo +0 77 V 31 E : +1-36 V Caloulate the AE° for the reaction 2t Fe(s) + Clacg) - te "(aq) t 2C1 (aq)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter13: An Introduction To Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 13.12QAP: The equilibrium constant for the reaction 2CrO42+2H+Cr2O72+H2O is 4.2 1014. The molar...
Related questions
Question
100%
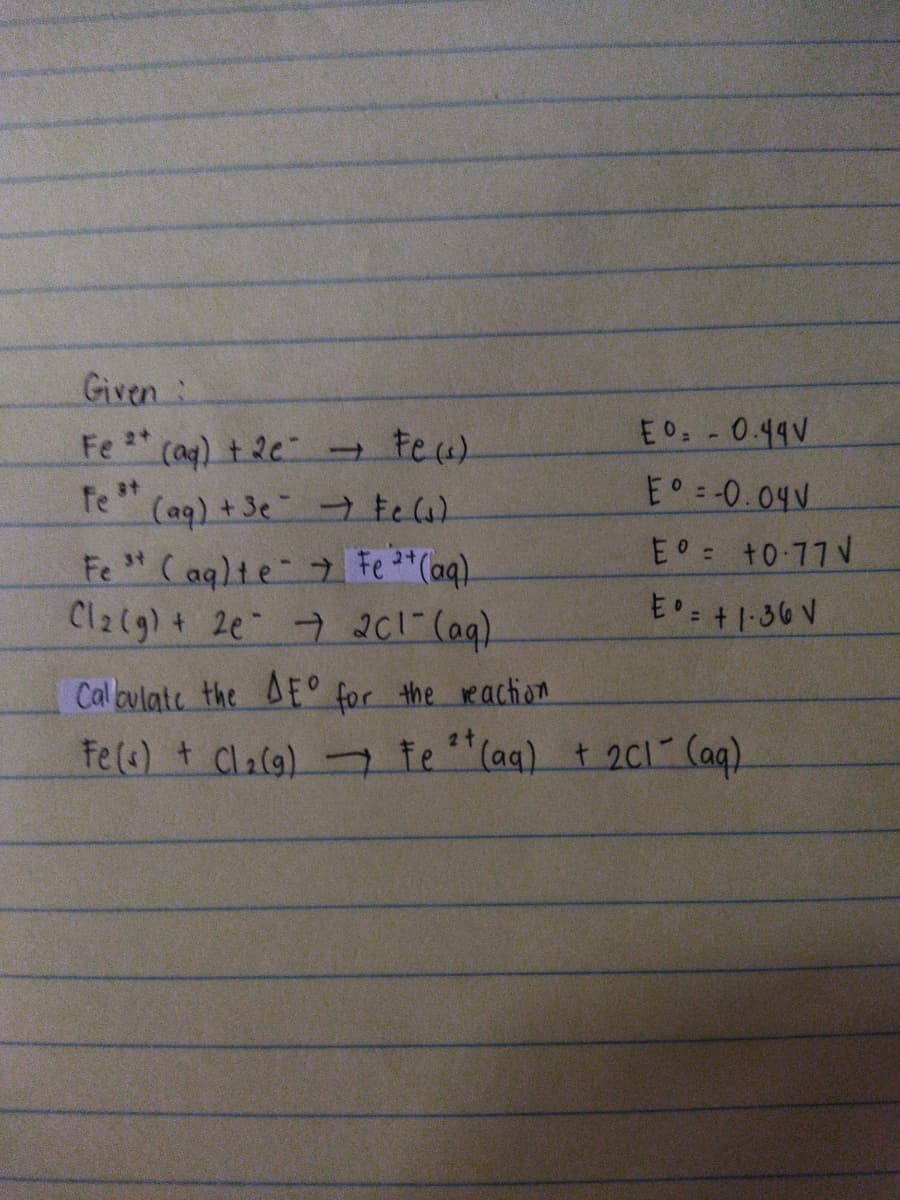
Transcribed Image Text:Given:
E0 -0.49V
Fe (ag) t 2e teu)
fe *"
E -0.04V
(aq) +3e tela)
E +0 77 V
Fe " (aqlte Fe "(aq).
Cl2(g) + 2e 7 201 (aq).
34
E': +1-36 V
Cal oulate the AE° for the reaction
2t
Fe(s) t Claca) - te "(aq) t 2ci Caq).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
