Given that K, is 7.5x10-3 (aq) for R-NH3* (aq) + H20(1) <--> R-NH2(aq) + H3O* (where R - is a carbon containing side chain) Calculate the pKb for R-NH2(aq) + H20(1) <--> R-NH3* (aq) + OH" (aq)
Given that K, is 7.5x10-3 (aq) for R-NH3* (aq) + H20(1) <--> R-NH2(aq) + H3O* (where R - is a carbon containing side chain) Calculate the pKb for R-NH2(aq) + H20(1) <--> R-NH3* (aq) + OH" (aq)
Chapter26: Biomolecules: Amino Acids, Peptides, And Proteins
Section26.SE: Something Extra
Problem 50AP: The -helical parts of myoglobin and other proteins stop whenever a proline residue is encountered in...
Related questions
Question
Use at least 2 sig figs and no scientific notation
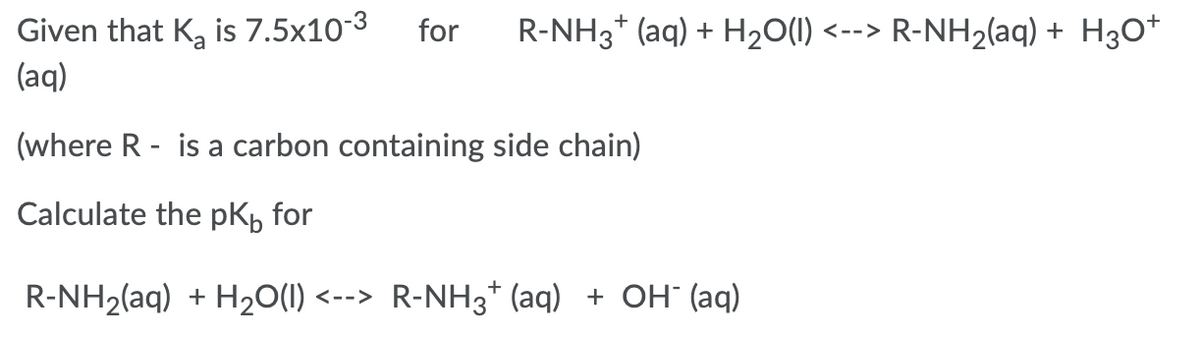
Transcribed Image Text:Given that K, is 7.5x10-3
(aq)
for
R-NH3* (aq) + H20(1) <--> R-NH2(aq) + H3O*
(where R - is a carbon containing side chain)
Calculate the pK, for
R-NH2(aq) + H20(1) <--> R-NH3* (aq) + OH" (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



