The radioactive isotope iodine-131 is used in medical imaging as indicated on the table above. If 40.2 milligrams of iodine-131 is administered to a patient, how many milligrams are left in the body after 16.1 days? mg
The radioactive isotope iodine-131 is used in medical imaging as indicated on the table above. If 40.2 milligrams of iodine-131 is administered to a patient, how many milligrams are left in the body after 16.1 days? mg
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter19: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 2RQ
Related questions
Question
7.3
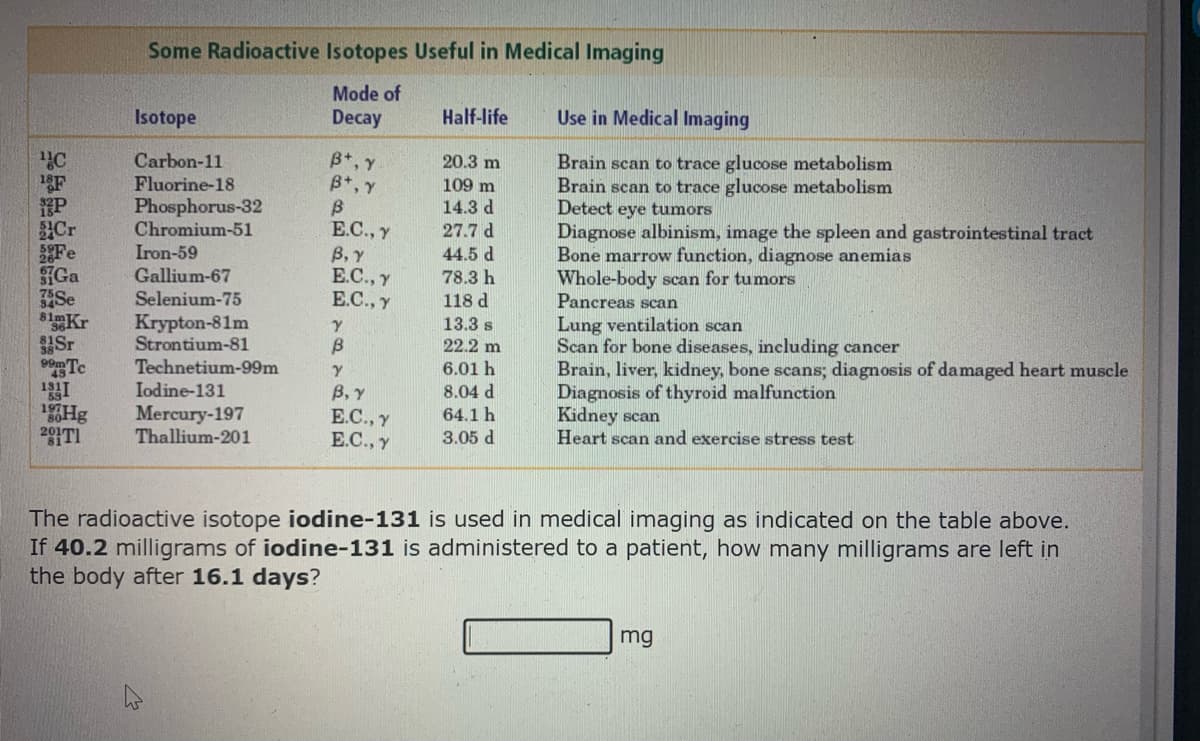
Transcribed Image Text:Some Radioactive Isotopes Useful in Medical Imaging
Mode of
Decay
Isotope
Half-life
Use in Medical Imaging
B+, Y
B*, Y
Carbon-11
20.3 m
F
P
Cr
Fe
SGa
Se
81mKr
Sr
Te
Brain scan to trace glucose metabolism
Brain scan to trace glucose metabolism
Detect eye tumors
Diagnose albinism, image the spleen and gastrointestinal tract
Bone marrow function, diagnose anemias
Whole-body scan for tumors
Pancreas scan
Lung ventilation scan
Scan for bone diseases, including cancer
Brain, liver, kidney, bone scans; diagnosis of damaged heart muscle
Diagnosis of thyroid malfunction
Kidney scan
Heart scan and exercise stress test
Fluorine-18
Phosphorus-32
Chromium-51
109 m
14.3 d
27.7 d
E.C., y
B, Y
Е.С., у
E.C., y
Iron-59
Gallium-67
44.5 d
78.3 h
Selenium-75
118 d
Krypton-81m
Strontium-81
Technetium-99m
Iodine-131
Mercury-197
Thallium-201
13.3 s
22.2 m
6.01 h
В, у
E.C., Y
E.C., y
8.04 d
64.1 h
291TI
3.05 d
The radioactive isotope iodine-131 is used in medical imaging as indicated on the table above.
If 40.2 milligrams of iodine-131 is administered to a patient, how many milligrams are left in
the body after 16.1 days?
mg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning