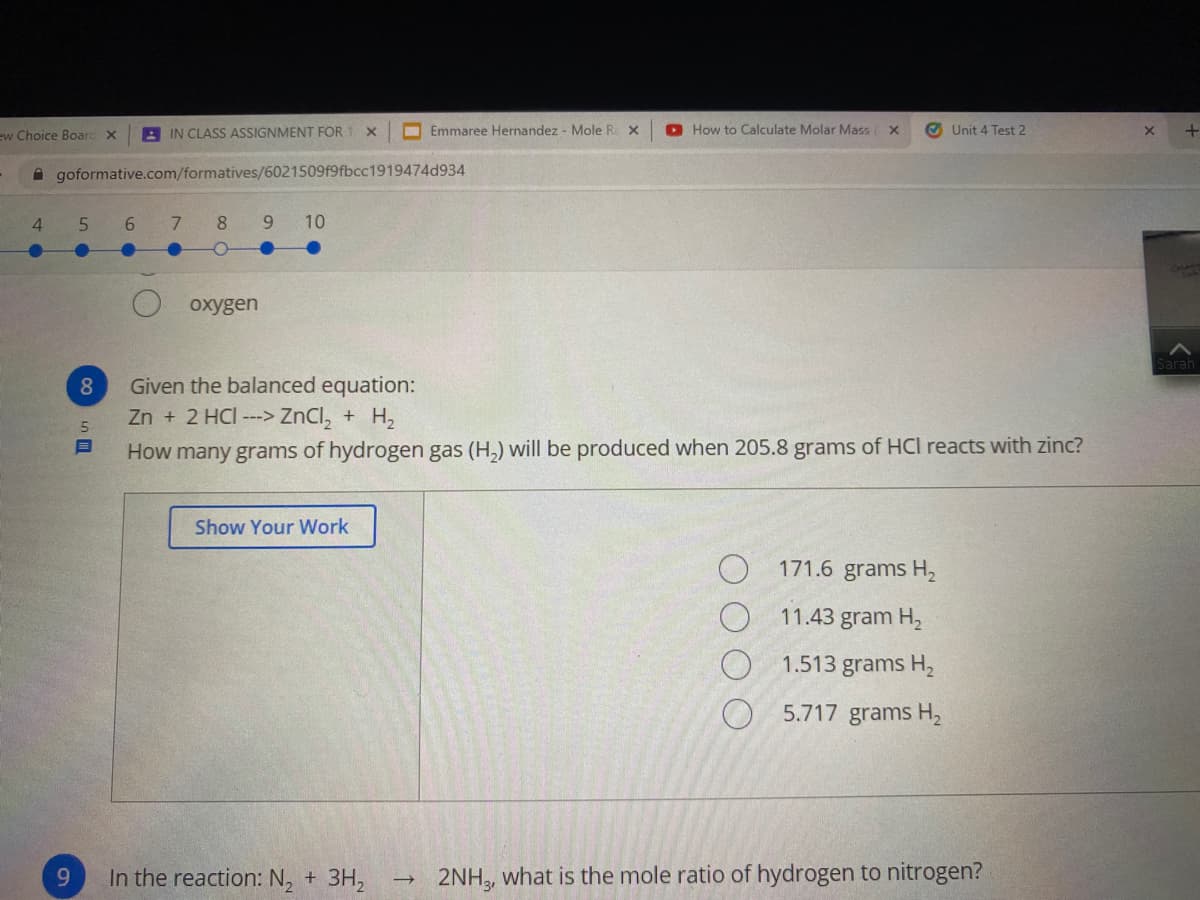
Given the balanced equation: Zn + 2 HCI ---> ZnCl, + H2 How many grams of hydrogen gas (H,) will be produced when 205.8 grams of HCI reacts with zinc?
Given the balanced equation: Zn + 2 HCI ---> ZnCl, + H2 How many grams of hydrogen gas (H,) will be produced when 205.8 grams of HCI reacts with zinc?
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.58E
Related questions
Question
Transcribed Image Text:ew Choice Boarc X
A IN CLASS ASSIGNMENT FOR
O Emmaree Hernandez - Mole R x
O How to Calculate Molar Mass x
O Unit 4 Test 2
+
A goformative.com/formatives/6021509f9fbcc1919474d934
4.
7.
9 10
oxygen
Sarah
Given the balanced equation:
Zn + 2 HCI ---> ZnCl, + H2
5.
How many grams of hydrogen gas (H,) will be produced when 205.8 grams of HCI reacts with zinc?
Show Your Work
171.6 grams H2
11.43 gram H,
1.513 grams H2
5.717 grams H2
9.
In the reaction: N, + 3H,
2NH, what is the mole ratio of hydrogen to nitrogen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

