Urea (CH,N,0) is a common fertilizer that is synthesized by the reaction of ammonia (NH3) with carbon dioxide: 2 NH3(aq) + CO2(ag)– CH,N;O(ag) + H,0(1) In an industrial synthesis of urea, a chemist combines 136.4 kg of ammonia with 211.4 kg of carbon dioxide and obtains 168.4 kg of urea. Determine the limiting reactant, theoretical yield of urea, and percent yield for the reaction.
Urea (CH,N,0) is a common fertilizer that is synthesized by the reaction of ammonia (NH3) with carbon dioxide: 2 NH3(aq) + CO2(ag)– CH,N;O(ag) + H,0(1) In an industrial synthesis of urea, a chemist combines 136.4 kg of ammonia with 211.4 kg of carbon dioxide and obtains 168.4 kg of urea. Determine the limiting reactant, theoretical yield of urea, and percent yield for the reaction.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.141QP: A power plant is driven by the combustion of a complex fossil fuel having the formula C11H7S. Assume...
Related questions
Question
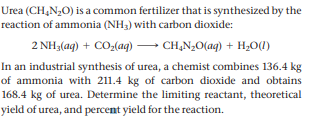
Transcribed Image Text:Urea (CH,N,0) is a common fertilizer that is synthesized by the
reaction of ammonia (NH3) with carbon dioxide:
2 NH3(aq) + CO2(ag)– CH,N;O(ag) + H,0(1)
In an industrial synthesis of urea, a chemist combines 136.4 kg
of ammonia with 211.4 kg of carbon dioxide and obtains
168.4 kg of urea. Determine the limiting reactant, theoretical
yield of urea, and percent yield for the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning