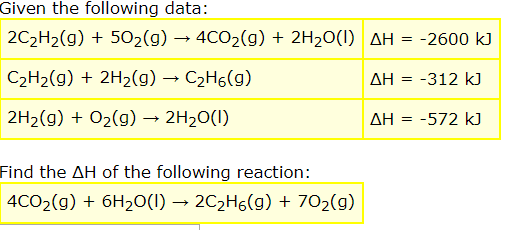
Given the following data: 2C2H2(g)502(g) 4CO2(g) + 2H20(I) AH = -2600 kJ C2H2(g)2H2(g) C2H6(g) AH = 312 k) 2H2(g)O2g) 2H20(I) AH = -572 k) Find the AH of the following reaction: 4CO2(g)6H20(I) - 2C2H6(g) 702(g)
Q: 7- Given the following data: 2H2 (g) + C (s) → CH4 (g) 2H2 (g) + O2 (g)→ 2H2O (I) C (s) + O2 (g) →…
A: The required equation is : The given chemical equations are:
Q: P4(s) + 6Cl2(g) → 4PCl3(g) -1225.6 II. P4(s) + 5O2(g) → P4O10(s) -2967.3 III. PCl3(g) + Cl2(g)…
A:
Q: Calculate the ΔG° (kJ/mol) for the following reaction at 152 °C, using the following data. Eneter…
A: Answer: -2085.472485 kJ/mole is the value of ∆G°rxn at 152°C
Q: Consider the problem below: (Equation 1) 5 CO(g) + 5 H2O(1) · C5H10(1) + 5 O2(g) AH = 890.6 kJ --->…
A:
Q: Calculate ΔH for the reaction: C6H6(l) + O2(g) à 6CO2(g) + 3H2O(l) given the following…
A: Given that: To find: ∆H for the given reaction =?
Q: How do I solve this
A:
Q: Given the following data: Pals) + 6 Clzig) → 4PC|3(9) AH = -1225.6 kJ Pa(s) + 5 Oz(g) → P4O10/s) AH…
A: enthalpy is an extensive property which will depend on the number of particle.
Q: THERMODYNAMICS: Calculate the value of AH (in kJ) for the reaction, 2AR3 (g) +Q (s) - A2R4 (g) + QR2…
A:
Q: Nitric acid, HNO3, was first prepared 1200 years ago by heating naturally occurring sodium nitrate…
A:
Q: Calculate Δ Hrxn for 2NOCl(g) → N2(g) + O2(g) + Cl2(g) given the following set of…
A: Given,12N2(g)+12O2(g)→NO(G), ∆H=90.3KJ -(1)NO(g)+12Cl2(g)→ NOCl(g),…
Q: Calculate AH for the reaction below using Hess' law. C2H6(g) → C2H2(g) + H2(g) AH=? C2H2(g)…
A:
Q: Calculate the AH for the reaction (6points) NO(g) + O(g) → NO2(g) given the following information:…
A: Heat of the reaction for the given reaction can be easily found by adding these reaction such that…
Q: 7- Given the following data: 2H2 (g) + C (s) → CH4 (g) 2H2 (g) + O2 (g)→ 2H2O (I) C (s) + O2 (g) –→…
A: Given: 2H2 (g) + C(s) ----> CH4 (g) Delta G1o = -51 kJ 2H2 (g) + O2 (g) ----> 2H2O…
Q: 7- Given the following data: 2H2 (g) + C (s) → CH4 (g) 2H2 (g) + O2 (g) → 2H2O (I ) C (s) + O2 (g) →…
A: Given,
Q: THERMODYNAMICSs: Calculate the value of AH (in kJ) for the reaction, 2AR3 (9) + Q (s) - A2R4 (g) +…
A: According to Hess's Law, Various chemical equations can be treated like algebraic equations and can…
Q: Acetylene burns in air according to the following equation: C2H2(g) + 5/2 O2(g) →2 CO2(g) +…
A: The standard free energy of formation refers to the energy change that occurs when a compound is…
Q: Calculate the value of ΔHo for the reaction 2 NO2 (g) --> N2O4 (g) at 298K Given ΔSo =…
A: Given chemical reaction :- 2 NO2 (g) --> N2O4 (g) At 298K ΔSo = -176 J/Kmol and ΔGo =…
Q: Use the following reactions to calculate the unknown AH. Fe203(s) + 3CO(g) → 2Fe(s) + 3CO2 (g) AH =…
A:
Q: Calculate AHrxn for the following reaction: 5C(s) + 6H2 (g) → C;H12 (1) Use the following reactions…
A: We are given 3 different reactions: C5H12(l)+8O2(g)-------->5CO2(g)+6H2O(g) H = - 3244.8…
Q: Given the following equation, C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) ΔG°rxn = -2074 kJ Calculate…
A: Consider the given target equation for which we have to determine the ΔG°rxn as; 27 CO2 (g) + 36 H2O…
Q: - (3.) At 25°C, the following heats of reaction are known: 2C¿H>(g) + 50½(8)→ 4CO2(g) + 2H2O(1) →…
A: The Heat of reaction is nothing but the standard enthalpy of reaction. This standard enthalpy of…
Q: Acetylene burns in air according to the following equation. C2H2(g) + 5/2 02(g) → 2 CO2(g) + H20(g)…
A:
Q: Use the standard reaction energies given below to determine AG*rxn for the following reaction:…
A:
Q: Determine AG rxn in kJ for 3X(g) + 2Y(g) = Z(g) + 3M(g) given the following information. Substance X…
A:
Q: Given : (a) BC13(2) + 3H2O¶→H;BO3(s) + 3HClg) AH = -112.5 kJ (b) B2H6(g) + 6H2O→2H3BO3(s) + 6H2(g)…
A: The reactions given are,
Q: 7- Given the following data: 2H2 (g) + C (s) –→ CH4 (g) 2H2 (g) + O2 (g) → 2H2O (I) C (s) + O2 (g) →…
A: Given, The reaction with unknown free energy change is - Add the equation (2) and (3) and subtract…
Q: Nitroglycerine is a shock-sensitive compound that decomposes as follows: 2 C3H5N3O9(l) -->…
A: The mass of nitroglycerine is 10.0 kg which is equal to 10.0 × 103 kg. The number of moles of…
Q: Show how the equations listed below with dHo values can be manipulated to calculate the dHo of the…
A:
Q: Consider the following reaction at 25 °C: 3 NiO(s) + 2 NH3(g) → 3 Ni(s) + N2(g) + 3 H20(g) If AG° =…
A:
Q: Stearic acid (C18H3602) is a fatty acid, a molecule with a long hydrocarbon chain and an organic…
A: Combustion Reaction: These are the reactions in which hydrocarbon reacts in the presence of oxygen…
Q: H2 (g) + 20 4. Calculate DH for the reaction 2 Al (s) +3 C12 (g)2 AIC13 (s) from the following data.…
A:
Q: Calculate the AH° for the reaction: 2C2H6 (g) + 7 O2 (g) 4CO2 (g) + 6H2O (g) Using the following…
A: Target equation is : 2C2H6(g) + 7O2(g) = 4CO2(g) + 6H2O(g) Applying Laplace-Lavoisier law and…
Q: Given the following data 2 CIF (g) + O2 (9) → Cl2 O(g) + F20(g) 2 CIF3 (g) + 202(9) → Cl2 O(g) +…
A: ClF(g) + F2(g) --> ClF3(g)
Q: I. CO (g) → C (s) + ½ O2 (g) AH= +110kJ II. CO (g) + ½ O2(g) CO2 (g) AH= -280kJ III. Fe2O3 (k) + 3CO…
A: Given Reactions- CO(g) → C(s) + 12O2(g) ∆H= +110 kJ ----(1)CO(g)…
Q: Calculate ΔHrxn for 2NOCl(g) → N2(g) + O2(g) + Cl2(g) given the following set of reactions: 1/2…
A:
Q: (Equation 4) 2 N2(g) + 5 O2(g) 2 N205(g) AH = ??????? > --- To find AH for equation 4 you must:…
A: Interpretation: We have to find enthalpy change for the final reaction.
Q: Calculate AHxn for the following reaction: 2C2H4 (g) + 2H2 (g) 2C2H6 (g) Using the following data…
A:
Q: 4C(s) + 4H2(g) + O2(g) → CH3CH2OCOCH3(l) ΔH°=-480.0 kJ CH3CH2OH(l) + O2(g) → CH3COOH(l) + H2O(l)…
A: This question belong to thermochemistry. In this reaction the standard enthalpy of the following…
Q: Consider the reaction A →B with AH° = 43.19 kJ/molxn and the reaction B → C with AH° = 81.98…
A: We know that according to Hess's law, the heat of reaction for a given reaction is the sum of the…
Q: Calculate AHrxn for the following reaction: C(s) +H20(g)→CO(g) +H2 (g) Use the following reactions…
A:
Q: 18. Calculate the AH of reaction for CIF(g)F2(g)> CIF3(g) given the following data: 1) 2CIF(g)O2(g)…
A:
Q: Which system does more work? O AE=650J & q =770J O AE= -410 J & q = - 100 J O AE=317 J & q = 347 J O…
A: According to the first law of thermodynamics, ∆E = q + w∆E = Change in internal energyq = Heat…
Q: At 25°C, the following heats of reaction are known: 2CIF(g) + O₂(g) Ⓡ Cl₂O(g) + F₂0 2CIF3(g) +202(g)…
A:
Q: hat is the molar energy of combustion (in kJ/mol) of C7H6O2 when 2.50 g of H6O2 burns completely in…
A: Given Reaction:- 2C7H6O2 + 15O2 -----> 14CO2 + 6H2O Mass of C7H6O2 = 2.50g Molar Mass of C7H6O2…
Q: 5. Given the following data: + %O2(g) B2H6(g) + 302(g) + ½O2(g) H2O(1) 2B(s) B2O3(s) AH = -1273…
A: To get the desired reaction from the given data , we have to adjust the given reaction . If we…
Q: Given the following data: 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(l) ΔG0= -6399 kJ…
A: Finding ∆G°
Q: the following equations (1-3) C(s) + 2 H₂(g) → CH₂(g) - values, determine the heat or reaction at…
A:
Q: a. From a consideration of the following reactions, calculate AH; for methane, CH4 (g). (1) C(g) +…
A: a. The equation for formation of methane is,
Q: Acetylene burns in air according to the following equation. C2H2(g) + 5/2 O2(g) 2 CO2(g) + H2O(g)…
A: The solution is given below -
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- Given the following data: 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(l) ΔG0= -6399 kJ C(s) + O2(g) → CO2(g) ΔG0= -394 kJ H2(g) + ½O2(g) → H2O(l) ΔG0= -237 kJ Calculate the ΔG0rxn for the reaction 6 C(s) + 3 H2(g) → C6H6(l)Use the molar DHf° under the formulas to calculate DH°rxn for the equations as balanced: 1. 2B2H6(g) + 3CO2(g) --> 2B2O3(s) + 3CH4(g) ΔH°rxn = _______ kJ ΔH°f = +36 –394 –1274 –75 kJ/mol exo ? endo_thermic 2. 2P2O5 + 2CaC2 -->P4 + 2CaCO3 + 2CO2 ΔH°rxn = _______ kJ ΔH°f = –1505 –59 ___ –1207 –394 kJ/mol exo ? endo_thermic 3. 2Na2CrO4(s) + 10HCl(g) --> 4NaCl(s) + 3Cl2(g) + Cr2O3(s) + 5H2O(l) ΔH°f = –1342 –92 –411 ___ –1140 –286 kJ/mol ΔH°rxn = _______ kJ exo ? endo_thermicShow how the equations listed below with dHo values can be manipulated to calculate the dHo of the following equation 2KCl(s) + H2SO4 (l) à 2HCl (g) + K2SO4 (s) dHo = ? a. HCl (g) + KOH (s) à KCl (s) + H2O (l) dHo = - 200.0 kJ b. H2SO4 (l) + 2KOH (s) à K2SO4 (s) + 2 H2O (l)dHo = -340.0 kJ
- 2CH4(g) → C2H6(g) + H2(g) ΔHrxn = ? Calculate ΔHrxn using the data below. Include negative sign if appropriate. ΔHrxn (kJ) H2(g) + ½ O2(g) → H2O(ℓ) -285.8 CH4(g) + 2O2(g) → CO2(g) +2H2O(ℓ) -890.4 C2H6(g) + 7/2 O2(g) → 2CO2(g) + 3H2O(ℓ) -1559.90 Calculate AH for the following reaction, after it is properly balanced with smallest whole-number coefficients: rxn C,H%(g) + Ozg) → COz(g) + H,O(g) AH [C₂H6(g)] = -84.667 kJ/mol AH [CO₂(g)] = -393.5 kJ/mol AH [CO₂(aq)] = −412.9 kJ/mol AH [H₂O(g)]=-241.826 kJ/mol AH [H₂O()] = -285.840 kJ/mol f kJ [unbalanced]2C8H18(l) + 25 O2(g) = 16 CO2(g) + 18 H2O(l) The standard enthalpy of formation for H2O(g)is −241.82kJ/mol The standard enthalpy of formation for CO2(g)is −393.5kJ/mol The standard enthalpy of formation for C8H18(l) is −249.9kJ/mol 1)how much CO2 is released from 1 tank of gas? 2) there are 3000 students and employees. Further assume that they all drive, and on an average they consume 1 tank of gas each, every week to get to and from work. If every tank is 12 gallons (1 gallon = 3.785 L) can you estimate how much heat is transferred
- Given the following data: 4C(s) + 3H2(g) + 1/2O2(g) → HCOC(CH2)(CH3)(l) ΔH°=-139.0 kJ CH3CH2OH(l) + 1/2O2(g) → CH3CHO(l) + H2O(l) ΔH°=-204.0 kJ 2C(s) + 3H2(g) + 1/2O2(g) → CH3CH2OH(l) ΔH°=-278.0 kJ H2(g) + 1/2O2(g) → H2O(l) ΔH°=-286.0 kJ Calculate ΔH° for the reaction:2CH3CHO(l) → HCOC(CH2)(CH3)(l) + H2O(l)For a particular isomer of C8H18,C8H18, the combustion reaction produces 5093.7 kJ5093.7 kJ of heat per mole of C8H18(g)C8H18(g) consumed, under standard conditions. C8H18(g)+252O2(g)⟶8CO2(g)+9H2O(g)Δ?∘rxn=−5093.7 kJ/molC8H18(g)+252O2(g)⟶8CO2(g)+9H2O(g)ΔHrxn°=−5093.7 kJ/mol What is the standard enthalpy of formation of this isomer of C8H18(g)?What is the value of ΔG°rxn for the reaction: C --> 2A+BGiven: 2A+B --> C and ΔG°rxn = 260.5 kJ/mol
- What volume (in mL) of benzene (C6H6, d= 0.88 g/mL, molar mass = 78.11 g/mol) is required to produce 2.38 x 10^3 kJ of heat according to reaction below? Report your answer to the nearest integer Reaction: 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(g) ΔH°rxn = -6278 kJGiven the following enthalpies of combustion: (a) 2H2(g) + O2(g) --> 2H2O(g); DH = -483.6 kJ (b) 2C3H6(g) + 9O2(g) --> 6CO2(g) + 6H2O(g); DH = -3853.6 kJ (c) C3H8(g) + 5O2(g) --> 3CO2(g) + 4H2O(l); DH = -2043.7 kJ Calculate DH for the following reaction: (A 4) C3H6(g) + H2(g) --> C3H8(g) (A) -124.9 kJ (B) -366.7 kJ (C) -2293.5 kJ (D) -4262.3kJNitric acid, HNO3, was first prepared 1200 years ago by heating naturally occurring sodium nitrate (called saltpeter) with sulfuric acid to produce sodium bisulfate and collecting the vapors of HNO3 produced. Calculate ∆H°rxn for this reaction. ∆H°f[NaNO3(s)] = -467.8 kJ/mol; ∆H°f[NaHSO4(s)] = -1125.5 kJ/mol; ∆H°f[H2SO4(l)] = -814.0 kJ/mol; ∆H°f[HNO3(g)] = -135.1 kJ/mol.NaNO3(s) + H2SO4(l) => NaHSO4(s) + HNO3(g)



