Given the standard enthalpy changes for the following two reactions: (1) N2(g) + O2(g) → 2NO(g) (2) N2(g) +202 (9) → N2O4(9) AH° 181.8 kJ AH° = 9.2 kJ What is the standard enthalpy change for the following reaction? (3) 2NO(g) + O2(g) → N2O4 (9) ΔΗ° =? Standard enthalpy change = 8.836 kj
Given the standard enthalpy changes for the following two reactions: (1) N2(g) + O2(g) → 2NO(g) (2) N2(g) +202 (9) → N2O4(9) AH° 181.8 kJ AH° = 9.2 kJ What is the standard enthalpy change for the following reaction? (3) 2NO(g) + O2(g) → N2O4 (9) ΔΗ° =? Standard enthalpy change = 8.836 kj
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.116QP: Ethylene glycol, HOCH2CH2OH, is used as antifreeze. It is produced from ethylene oxide, C2H4O, by...
Related questions
Question
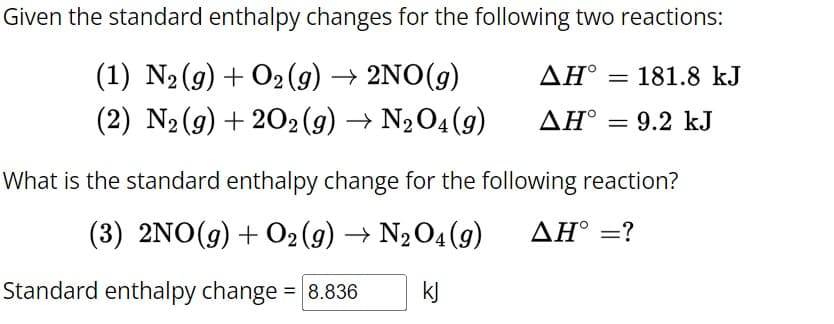
Transcribed Image Text:Given the standard enthalpy changes for the following two reactions:
(1) N2(g) + O2(g) → 2NO(g)
(2) N2(g) +202 (9) → N2O4(9)
AH° 181.8 kJ
AH° = 9.2 kJ
What is the standard enthalpy change for the following reaction?
(3) 2NO(g) + O2(g) → N2O4 (9)
ΔΗ° =?
Standard enthalpy change = 8.836
kj
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
