Given:(NH4)2SO4 + Ba(NO3)2 -->___NH4NO3 +___BaSO4 54.6g of ammonium sulfate reacts with 67.8g of barium nitrate. A) What is the limiting reactant? B) What mass of barium sulfate will be produced? Answer A): Limiting Reactant, Answer B): number unit of formula Answer A): *For chemical formulas: enter subscripts as normal number, and no spaces. << Previous 80 F3 JAN 24 Q F4 9 , Answer B): F5 tv c F6 K F7 A DII F8 A of N F9
Given:(NH4)2SO4 + Ba(NO3)2 -->___NH4NO3 +___BaSO4 54.6g of ammonium sulfate reacts with 67.8g of barium nitrate. A) What is the limiting reactant? B) What mass of barium sulfate will be produced? Answer A): Limiting Reactant, Answer B): number unit of formula Answer A): *For chemical formulas: enter subscripts as normal number, and no spaces. << Previous 80 F3 JAN 24 Q F4 9 , Answer B): F5 tv c F6 K F7 A DII F8 A of N F9
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 66QAP: Chlorine and fluorine react to form gaseous chlorine trifluoride. Initially, 1.75 mol of chlorine...
Related questions
Question
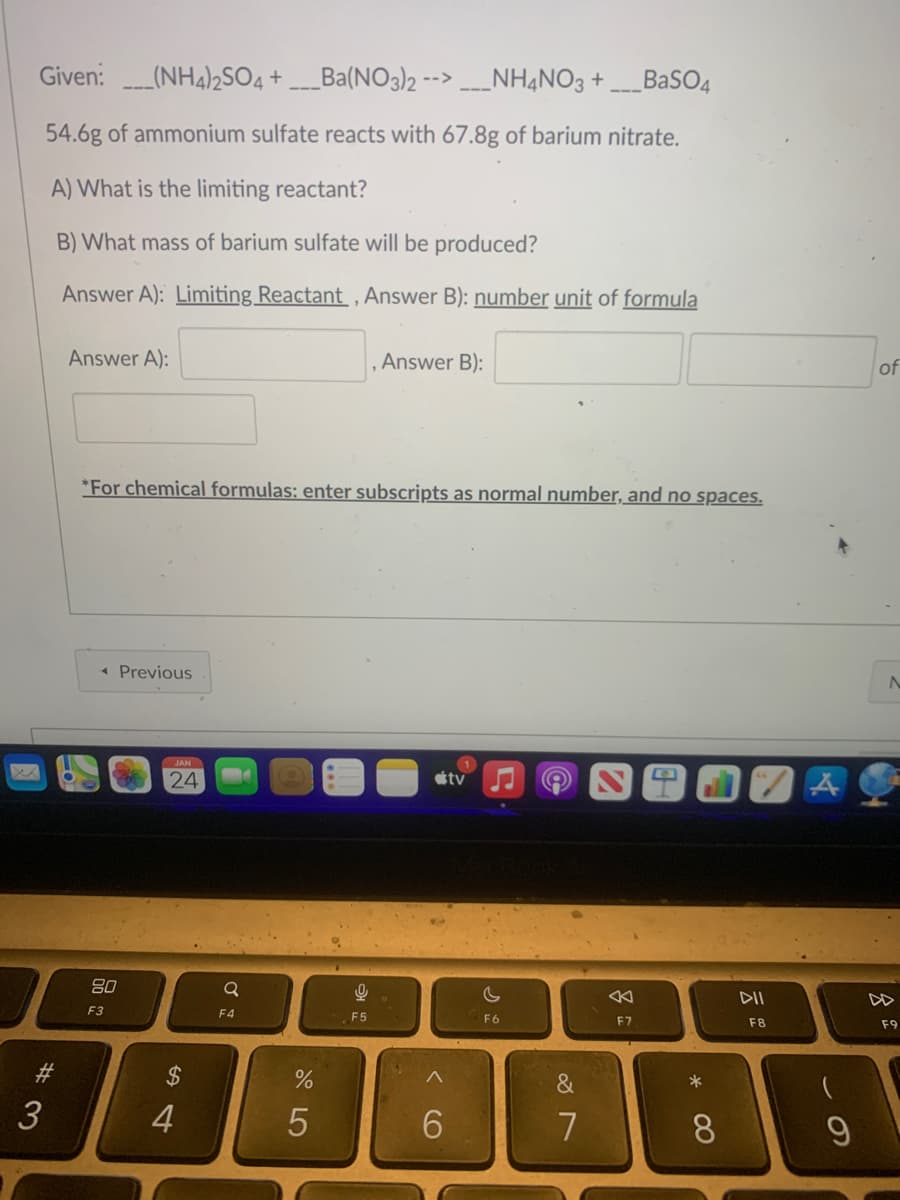
Transcribed Image Text:Given:(NH4)2SO4 +___Ba(NO3)2 -- ____NH4NO3 +___BaSO4
54.6g of ammonium sulfate reacts with 67.8g of barium nitrate.
A) What is the limiting reactant?
B) What mass of barium sulfate will be produced?
Answer A): Limiting Reactant, Answer B): number unit of formula
#3
Answer A):
*For chemical formulas: enter subscripts as normal number, and no spaces.
◄ Previous
80
F3
JAN
24
$
4
Q
F4
%
5
Answer B):
9
F5
6
1
tv
c
F6
&
7
F7
* 00
8
DII
F8
- O
9
of
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div