ground-state electron configuration: excited-state electron configuration: What is the energy difference in joules between energy sublevels 3s and 3p in a sodium atom? energy difference: How many of these electronic transitions must occur to produce 2.25 kJ of light energy? photons electronic transitions:
ground-state electron configuration: excited-state electron configuration: What is the energy difference in joules between energy sublevels 3s and 3p in a sodium atom? energy difference: How many of these electronic transitions must occur to produce 2.25 kJ of light energy? photons electronic transitions:
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 141QRT
Related questions
Question
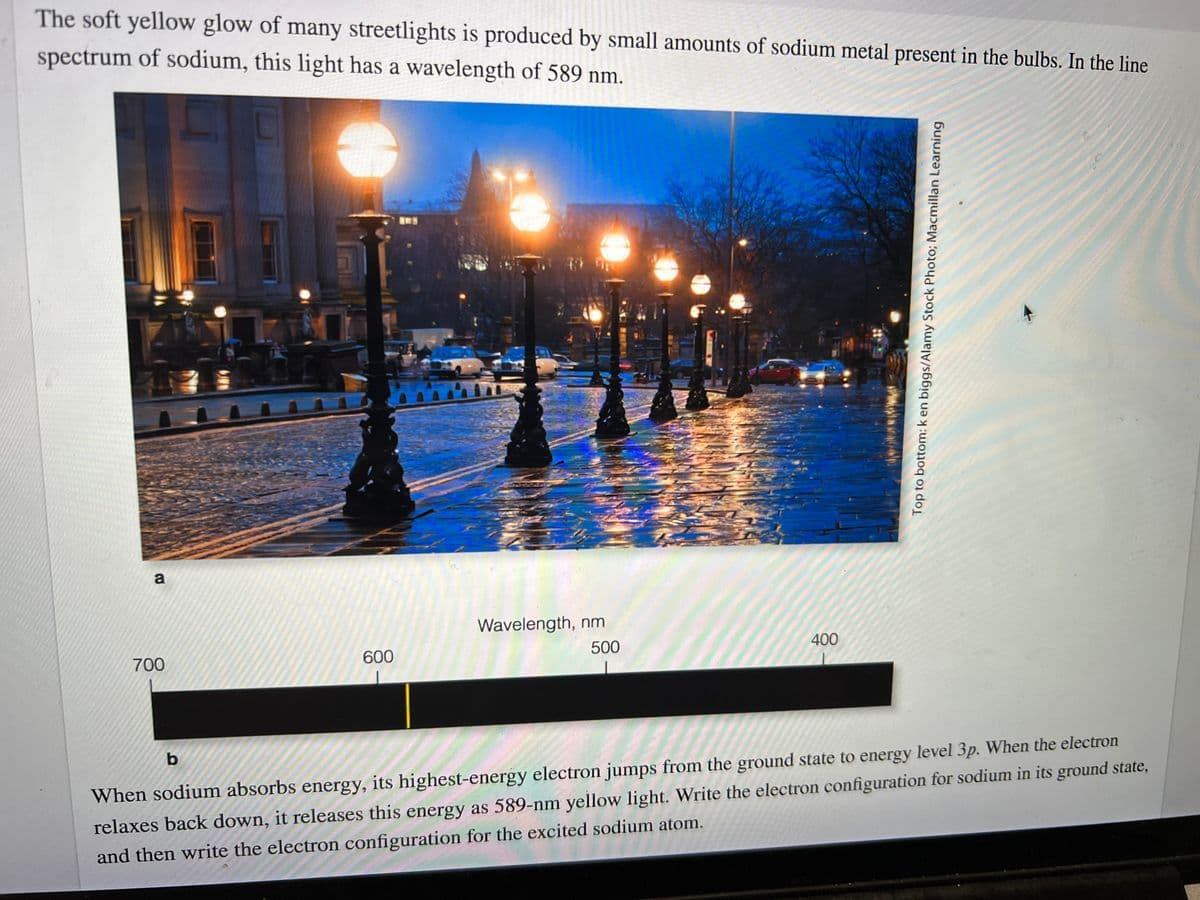
Transcribed Image Text:The soft yellow glow of many streetlights is produced by small amounts of sodium metal present in the bulbs. In the line
spectrum of sodium, this light has a wavelength of 589 nm.
ES
a
Wavelength, nm
500
400
700
600
When sodium absorbs energy, its highest-energy electron jumps from the ground state to energy level 3p. When the electron
relaxes back down, it releases this energy as 589-nm yellow light. Write the electron configuration for sodium in its ground state,
and then write the electron configuration for the excited sodium atom.
Top to bottom: k en biggs/Alamy Stock Photo; Macmillan Learning
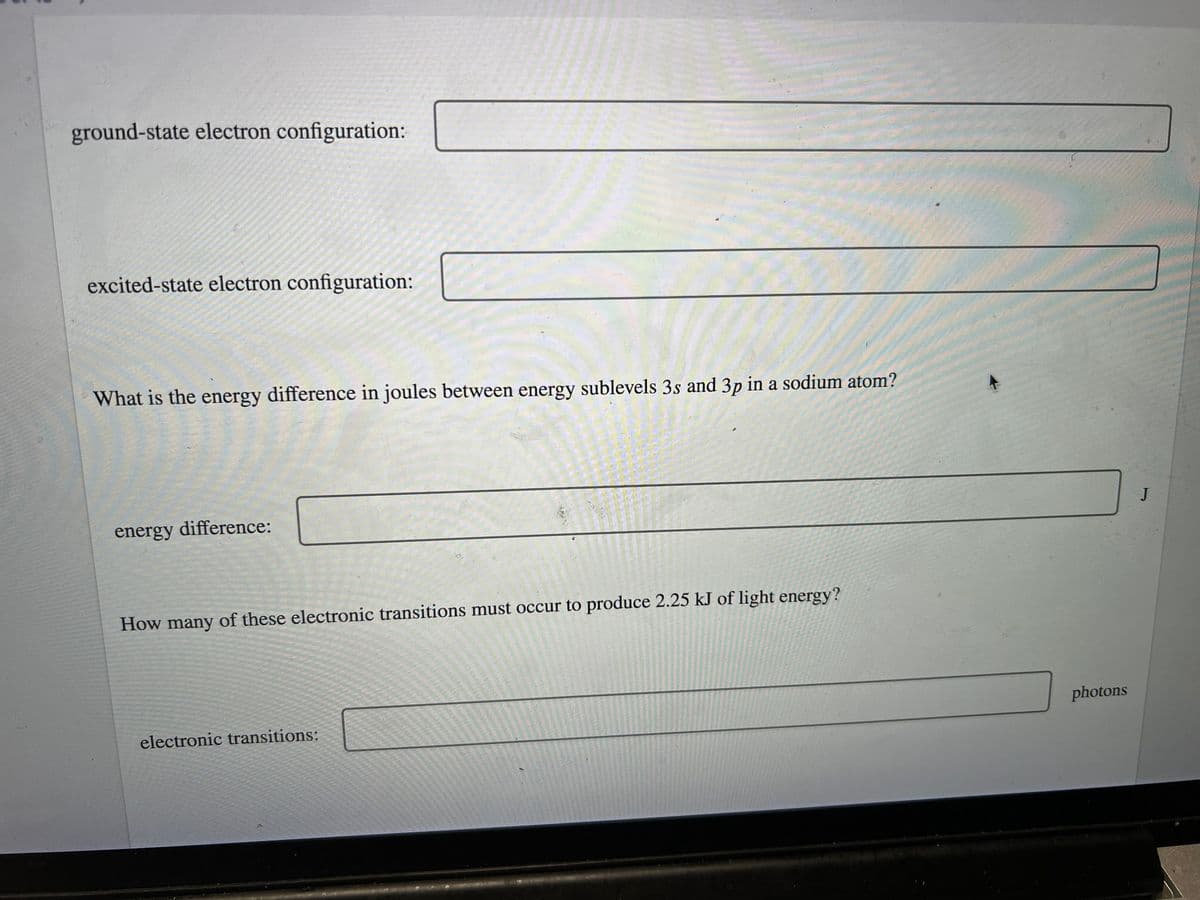
Transcribed Image Text:ground-state electron configuration:
excited-state electron configuration:
What is the energy difference in joules between energy sublevels 3s and 3p in a sodium atom?
J
energy difference:
How many of these electronic transitions must occur to produce 2.25 kJ of light energy?
photons
electronic transitions:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning