Identify the neutral element represented by this excited-state electron configuration. excited state: 1s 2s²2p°3s²3p³4s' element symbol: Write the full ground-state electron configuration for that element. ground state:
Identify the neutral element represented by this excited-state electron configuration. excited state: 1s 2s²2p°3s²3p³4s' element symbol: Write the full ground-state electron configuration for that element. ground state:
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 10P
Related questions
Question
I can't fit both sections of the question itto one shot, so please answer both parts. Thank you very much
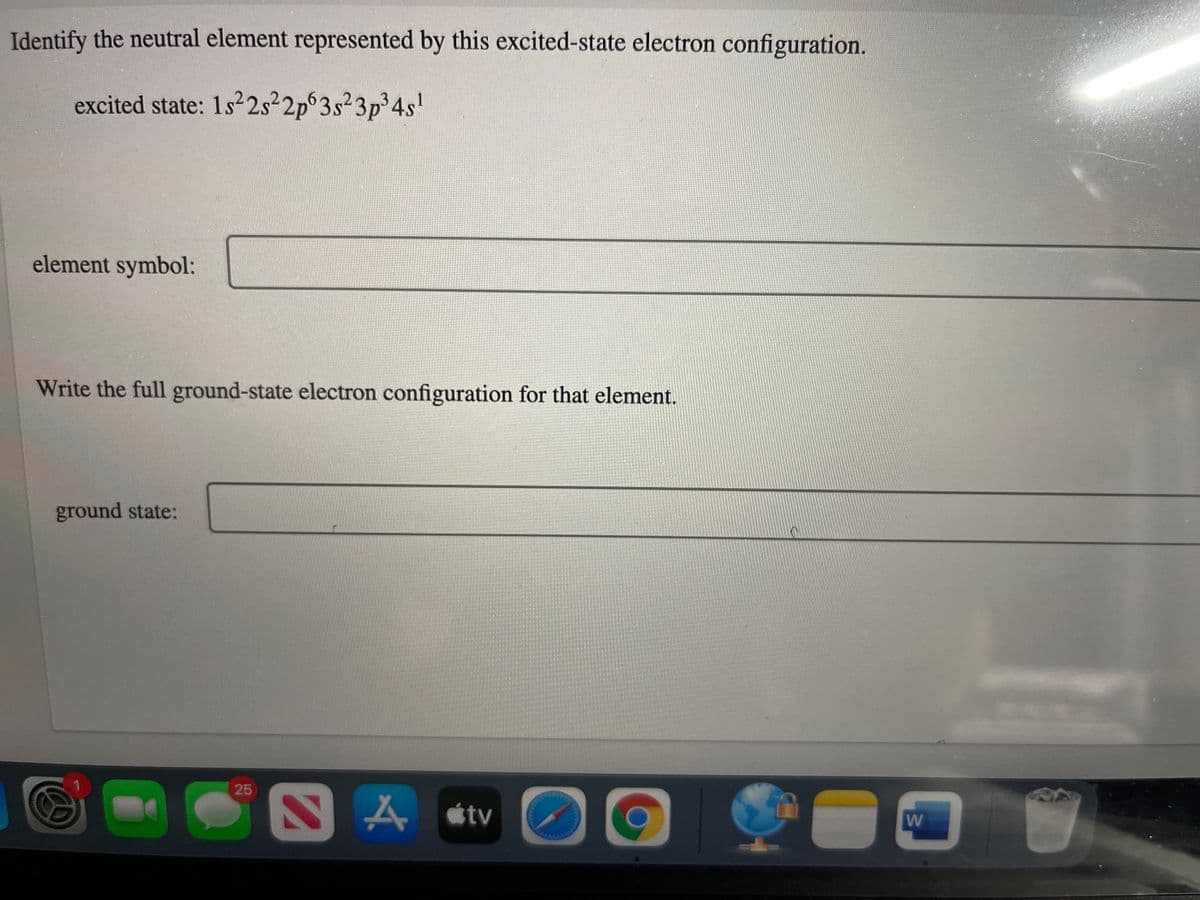
Transcribed Image Text:Identify the neutral element represented by this excited-state electron configuration.
excited state: 1s22s²2p 3s²3p³4s'
element symbol:
Write the full ground-state electron configuration for that element.
ground state:
25
SA stv
W
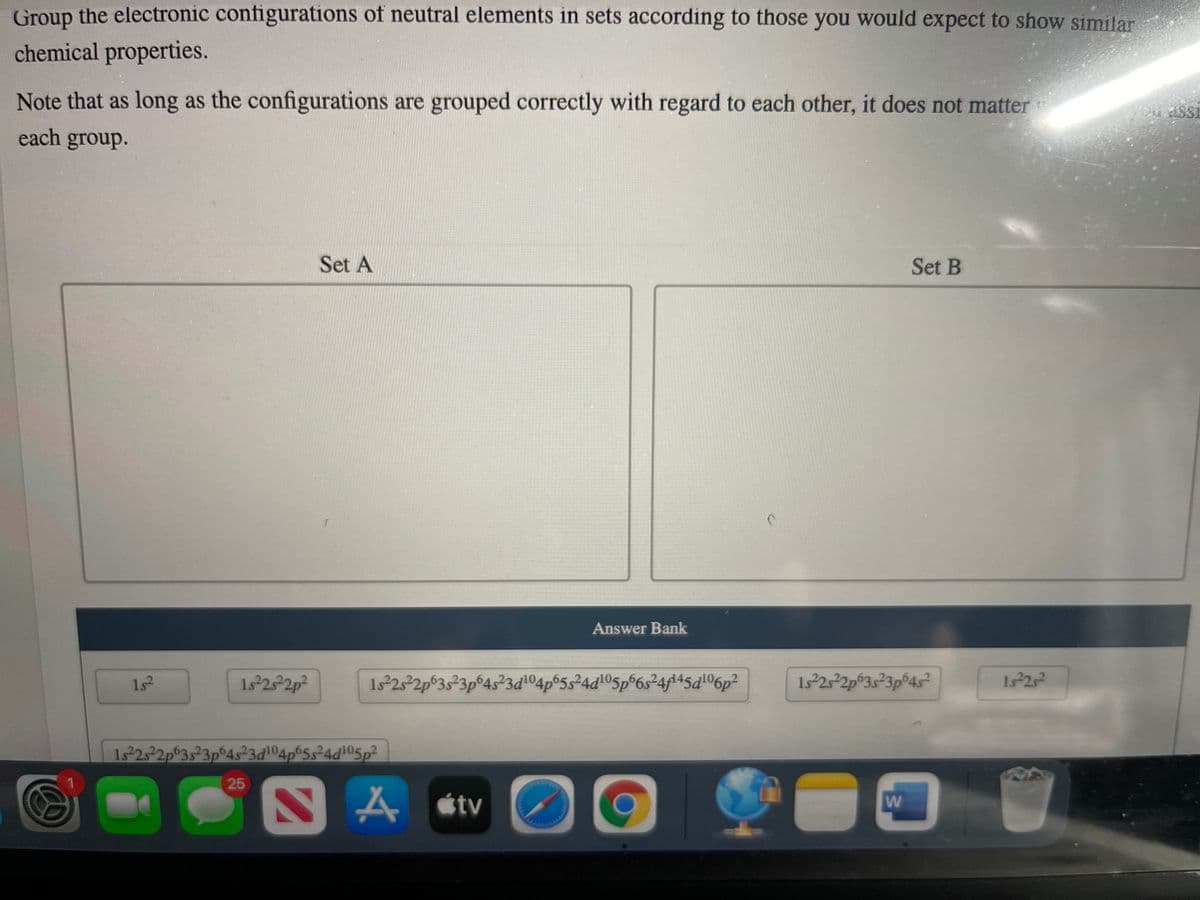
Transcribed Image Text:Group the electronic configurations of neutral elements in sets according to those you would expect to show similar
chemical properties.
Note that as long as the configurations are grouped correctly with regard to each other, it does not matter
each group.
Set A
Set B
Answer Bank
1s2
1. 252p?
1s25²2p°3s²3p©4s²3d104p®5s²4d!O5p°6s°4f145d!06p²
1s 25 2p°3s²3p®4s²
1s2s2
1s 252p°3523p°4s²3d104p65s²4d105p²
25
A étv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning