Group 1 & 2 Consider a steel cylinder with an inner height of h = 1.000 m and an inner diameter of d = 200.0 cm. Suppose this cylinder is filled completely with carbon disulfide when the temperature is 12. 5 °C. How much carbon disulfide will spill over when the temperature rises to 35.0 °C ?
Group 1 & 2 Consider a steel cylinder with an inner height of h = 1.000 m and an inner diameter of d = 200.0 cm. Suppose this cylinder is filled completely with carbon disulfide when the temperature is 12. 5 °C. How much carbon disulfide will spill over when the temperature rises to 35.0 °C ?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.16E: With reference to Joules apparatus inFigure2.6, assuminga massof 100.Kg ofwater about100L,a weight...
Related questions
Question
100%
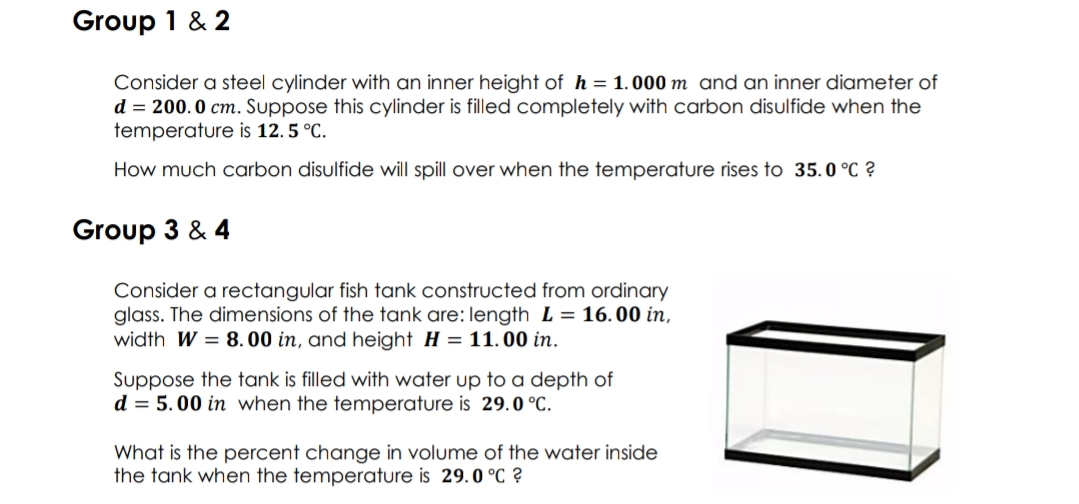
Transcribed Image Text:Group 1 & 2
Consider a steel cylinder with an inner height of h = 1. 000 m and an inner diameter of
d = 200.0 cm. Suppose this cylinder is filled completely with carbon disulfide when the
temperature is 12. 5 °C.
How much carbon disulfide will spill over when the temperature rises to 35.0 °C ?
Group 3 & 4
Consider a rectangular fish tank constructed from ordinary
glass. The dimensions of the tank are: length L = 16.00 in,
width W = 8.00 in, and height H = 11.00 in.
Suppose the tank is filled with water up to a depth of
d = 5.00 in when the temperature is 29.0 °C.
What is the percent change in volume of the water inside
the tank when the temperature is 29.0 °C ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning