Group A: Simple Molecules CH4 NH3 H20 SİF4 NCI3 Group B: Polyatomic Ions PO43- Cl03 ClO4 SO32- Group C: Multiple Bonds H2CO HCN CO CO2 Lewis Structure Worksheet #1 - Page 1
Group A: Simple Molecules CH4 NH3 H20 SİF4 NCI3 Group B: Polyatomic Ions PO43- Cl03 ClO4 SO32- Group C: Multiple Bonds H2CO HCN CO CO2 Lewis Structure Worksheet #1 - Page 1
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter1: Bond Angles And Shape
Section: Chapter Questions
Problem 16CTQ: How many central atoms does the molecule H2NCH3 have, and what is the shape about each?
Related questions
Question
100%
Instructions:
Using the Lewis Structures assignment, take each molecule and redraw with correct geometry.
Identify the name of the molecular geometry and the bond angle between the central atom and the surrounding atoms.
If there is more than one central atom, assign a geometry and bond angle for each.
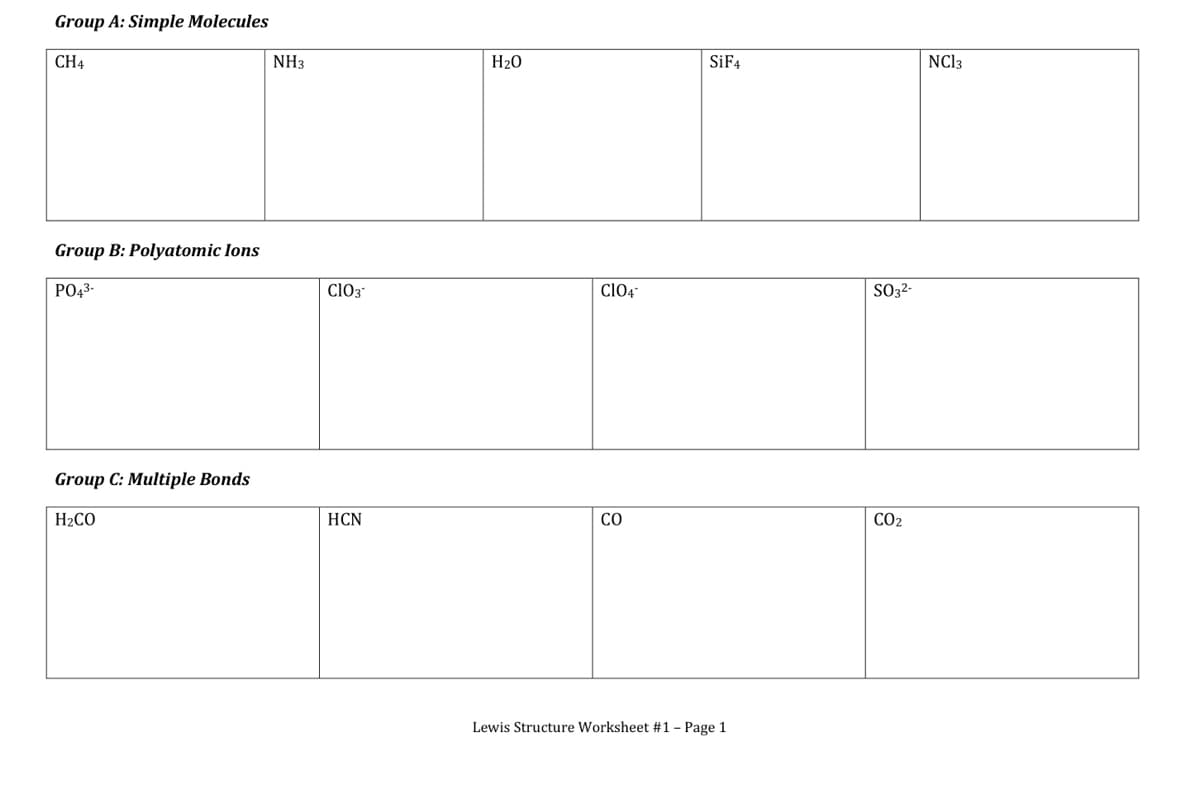
Transcribed Image Text:Group A: Simple Molecules
CH4
NH3
H20
SİF4
NCI3
Group B: Polyatomic Ions
PO43-
ClO3
ClO4-
SO32-
Group C: Multiple Bonds
H2CO
HCN
CO
CO2
Lewis Structure Worksheet #1 - Page 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
