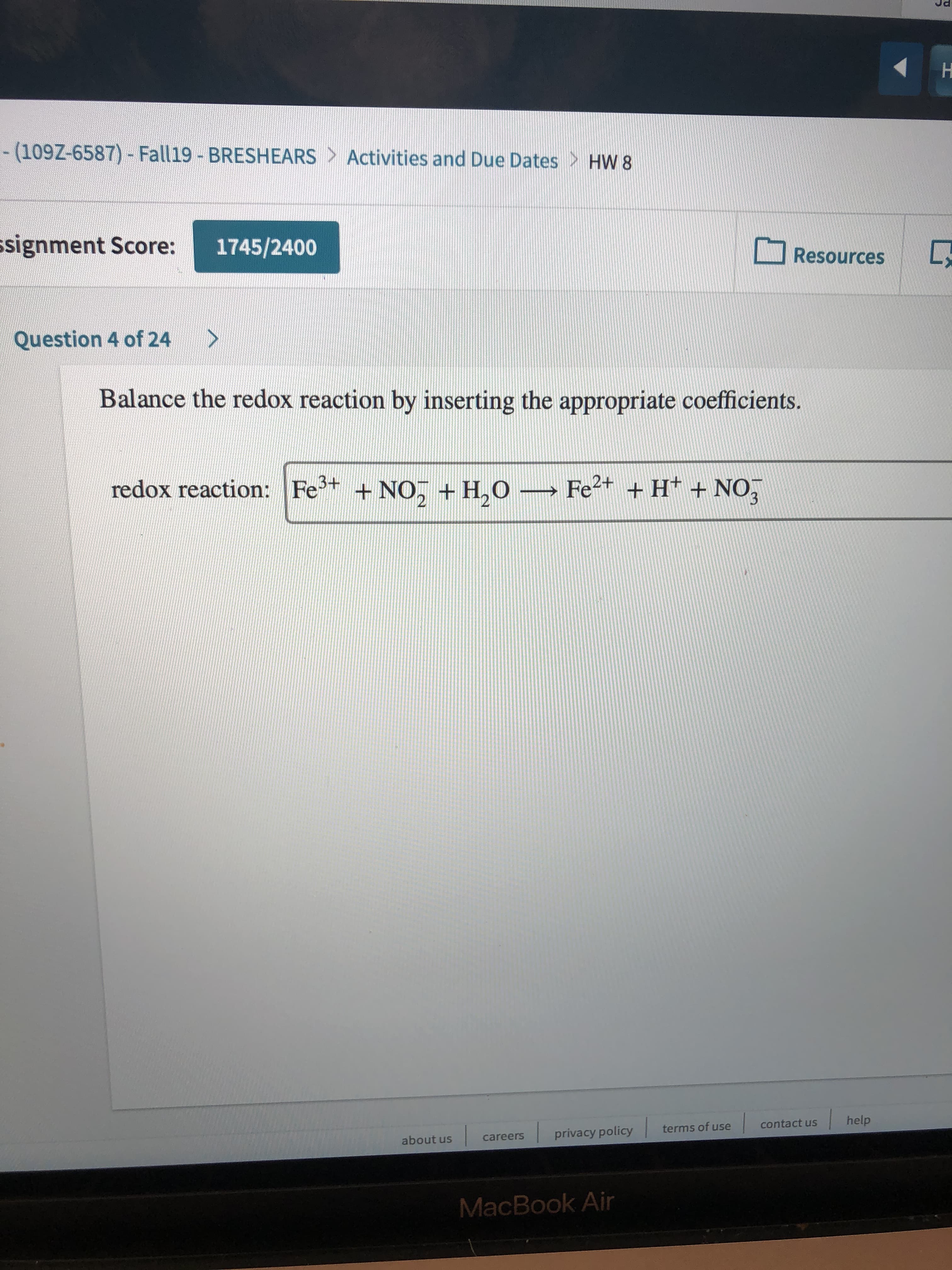
H - (109Z-6587)- Fall19 - BRESHEARS Activities and Due Dates HW 8 signment Score: 1745/2400 Resources > Question 4 of 24 Balance the redox reaction by inserting the appropriate coefficients redox reaction: Fe Fe2t + H +NO +NO, + H,O help contact us terms of use privacy policy careers about us MacBook Air
Science behind corrosion-test
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
Corrosion
Corrosion is defined as an activity that transforms refined metals into more chemically stable forms such as oxide, hydroxide, carbonate, or sulfide. It refers to the slow decomposition of things (typically metals); thanks to chemical and/or electrochemical reactions with their surroundings. Corrosion engineering is the science of preventing and controlling corrosion.
balance this
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images






