and Ecell for a redox reaction with n = 2 Calculate AG, that has an equilibrium constant of K 43x10-2. %3D Part A You may want to reference (Pages 861 865) Section 19.5 while completing this problem. Express your answer using two significant figures. η ΑΣΦ AG kJ Express your answer using two significant figures. E cell =
and Ecell for a redox reaction with n = 2 Calculate AG, that has an equilibrium constant of K 43x10-2. %3D Part A You may want to reference (Pages 861 865) Section 19.5 while completing this problem. Express your answer using two significant figures. η ΑΣΦ AG kJ Express your answer using two significant figures. E cell =
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 105QAP: Consider a voltaic cell in which the following reaction occurs. Zn(s)+Sn2+(aq)Zn2+(aq)+Sn(s) (a)...
Related questions
Question
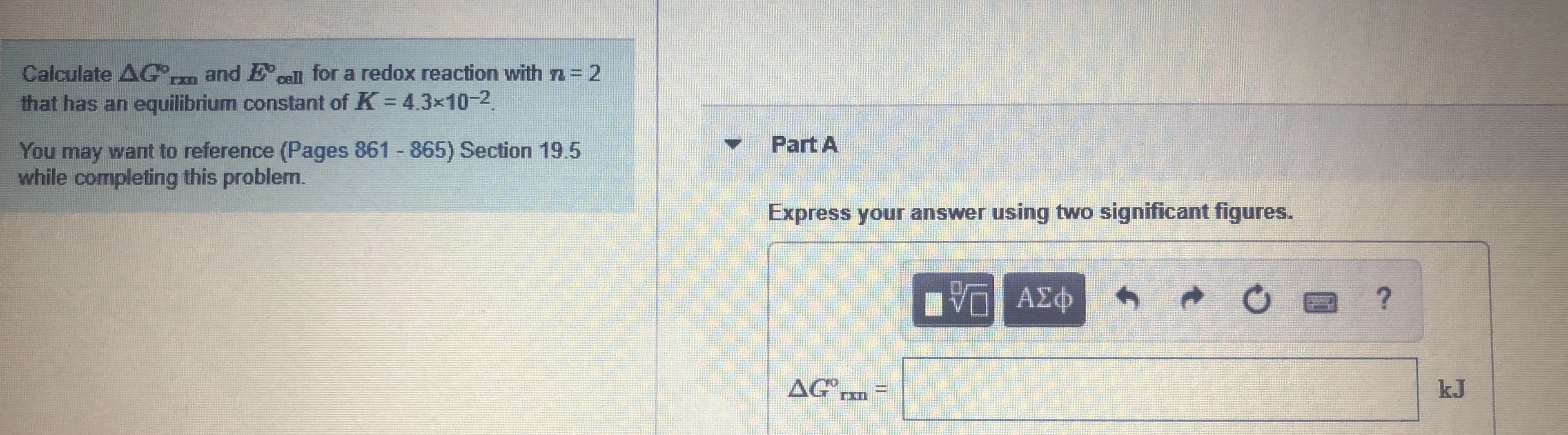
Transcribed Image Text:and Ecell for a redox reaction with n = 2
Calculate AG,
that has an equilibrium constant of K 43x10-2.
%3D
Part A
You may want to reference (Pages 861 865) Section 19.5
while completing this problem.
Express your answer using two significant figures.
η ΑΣΦ
AG
kJ
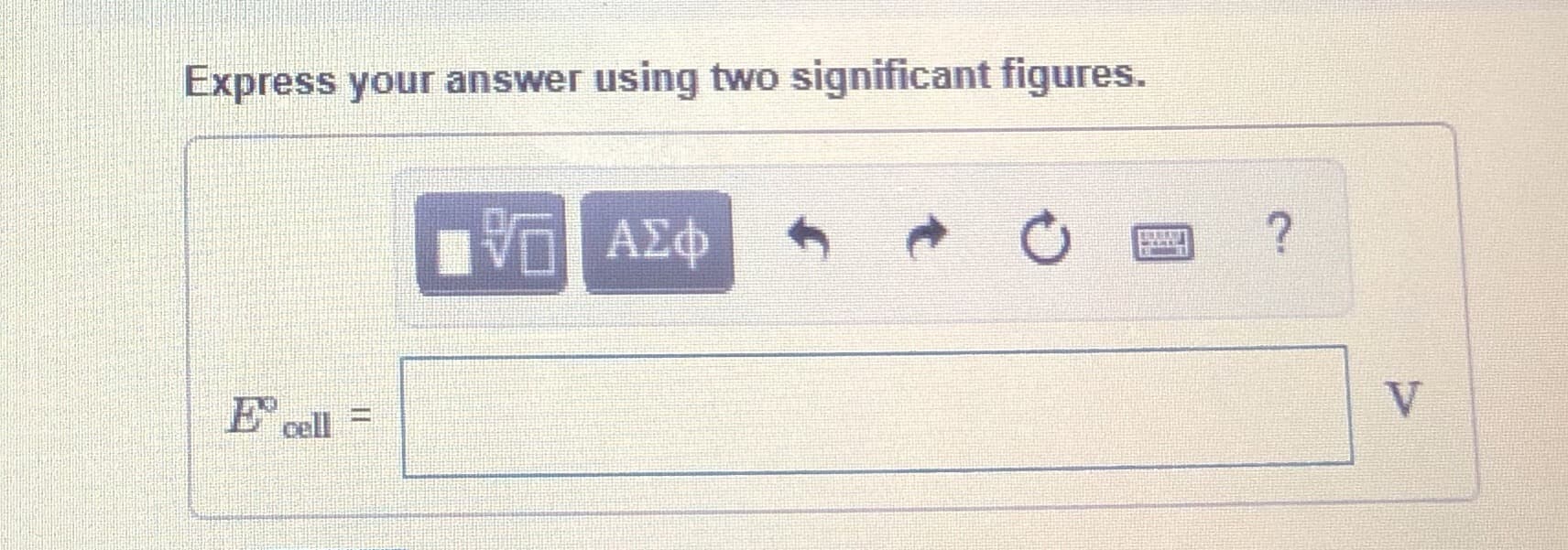
Transcribed Image Text:Express your answer using two significant figures.
E cell =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
