Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 68QAP: Consider the pyrosulfate ion, S2O72-. It has no sulfur–sulfur nor oxygen–oxygen bonds. (a) Write...
Related questions
Question
I had gotten this one incorrect, but i wanted to use it to study, so please give me the solution to all parts of this question.
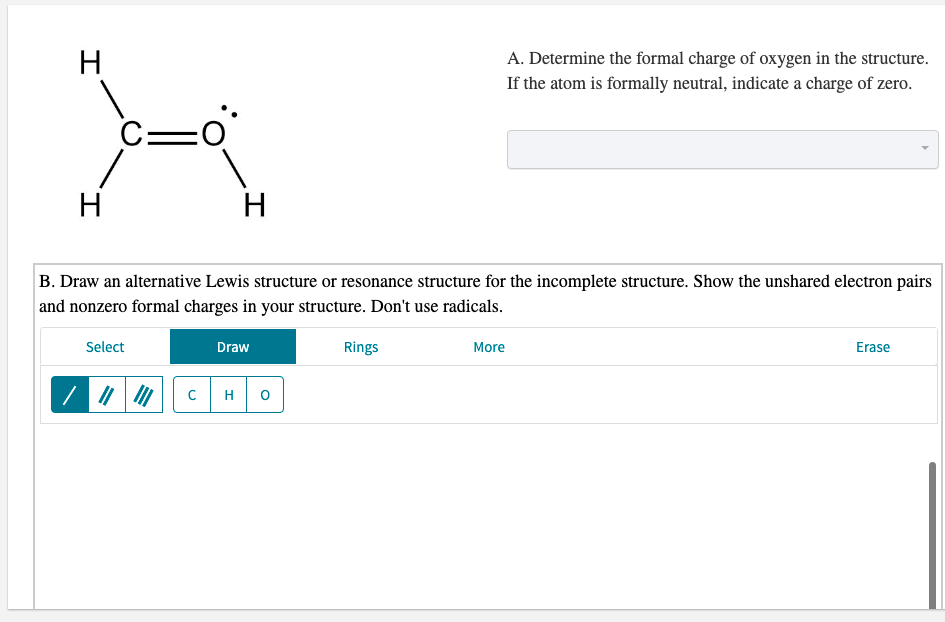
Transcribed Image Text:H
A. Determine the formal charge of oxygen in the structure.
If the atom is formally neutral, indicate a charge of zero.
C=
H
H
B. Draw an alternative Lewis structure or resonance structure for the incomplete structure. Show the unshared electron pairs
and nonzero formal charges in your structure. Don't use radicals.
Select
Draw
Rings
More
Erase
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning