Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following:
(a) The number of moles and the mass of chlorine, Cl2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl.
(b) The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of mercury(II) oxide.
(c) The number of moles and the mass of sodium nitrate, NaNO3, required to produce 128 g of oxygen. (NaNO2 is the other product.)
(d) The number of moles and the mass of carbon dioxide formed by the combustion of 20.0 kg of carbon in an excess of oxygen.
(e) The number of moles and the mass of copper(II) carbonate needed to produce 1.500 kg of copper(II) oxide. (CO2 is the other product.)
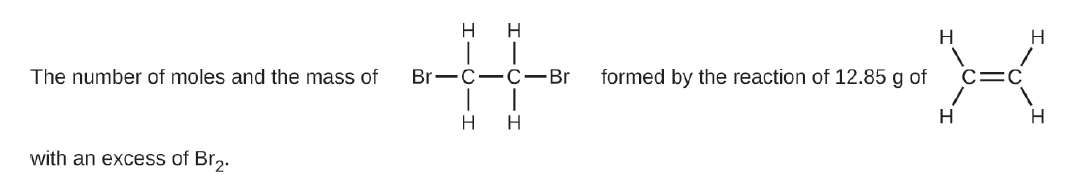
(f) See Image
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 8 images









