Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 2QAP: Your roommate, a chemistry major, claims to have synthesized the compound CH5 in the lab. Why is...
Related questions
Question
Answer #2 and #5.
Transcribed Image Text:X**
H.
H
HH
Н.
`H
H.
Η Η
CHA
C3H6
C3H3

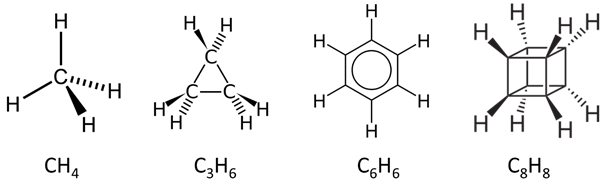
Transcribed Image Text:1. Which of the molecules does NOT have a horizontal mirror plane (oh) as its symmetry element?
A. CH4 B. C3H6 C. C6H6 D. C8H3 E. None of the above
2. Which of the following does NOT a have a C3 axis as its symmetry element?
A. CH4 B. C3H6 C. C6H6 D. CgHg E. None of the above
3. Which compound have the least number of symmetry elements in its structure?
A. CH4 B. C3H6 C. C6H6 D. CgHg E. None of the above
4. Which compound is expected to have the least number of molecular orbitals in its MO diagram?
A. CH4 B. C3H6 C. C6H6 D. C8H8 E. None of the above
5. Which of the following does NOT have the correct assignment according to its Schoenflies point group
classification?
A. CH4 : Td B. C3H6 : D3h C. C6H6 : D6h D. C8H8 : D4h E. None of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning