H HH Η Η Η H-C-C-C=H H-C-C-C-H ОН ОН ОН CI CI ČI Glycerol Trichloropropane Boiling point 290°C Boiling point 157°C The structural formulas of glycerol and trichloropropane are given above. Both compounds are liquids at 25°C. a. For each compound, identify all types of intermolecular forces present in the liquid. Explain why glycerol has the higher boiling point in terms of the relative strengths of the intemmolecular forces. b. Glycerol (molar mass 92.09 g/mol) has been suggested for use as an altemative fuel. The enthalpy of combustion, AH®comb , of glycerol is -1654 kJ/mol. What mass of glycerol would need to be combusted to heat 500.0 g of water from 20.0°C to 100.0°C ? (The specific heat capacity of water is 4.184 J/(g-°C). Assume that all the heat released by the combustion reaction is absorbed by the water.)
H HH Η Η Η H-C-C-C=H H-C-C-C-H ОН ОН ОН CI CI ČI Glycerol Trichloropropane Boiling point 290°C Boiling point 157°C The structural formulas of glycerol and trichloropropane are given above. Both compounds are liquids at 25°C. a. For each compound, identify all types of intermolecular forces present in the liquid. Explain why glycerol has the higher boiling point in terms of the relative strengths of the intemmolecular forces. b. Glycerol (molar mass 92.09 g/mol) has been suggested for use as an altemative fuel. The enthalpy of combustion, AH®comb , of glycerol is -1654 kJ/mol. What mass of glycerol would need to be combusted to heat 500.0 g of water from 20.0°C to 100.0°C ? (The specific heat capacity of water is 4.184 J/(g-°C). Assume that all the heat released by the combustion reaction is absorbed by the water.)
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter22: Organic And Biological Molecules
Section: Chapter Questions
Problem 176MP
Related questions
Question
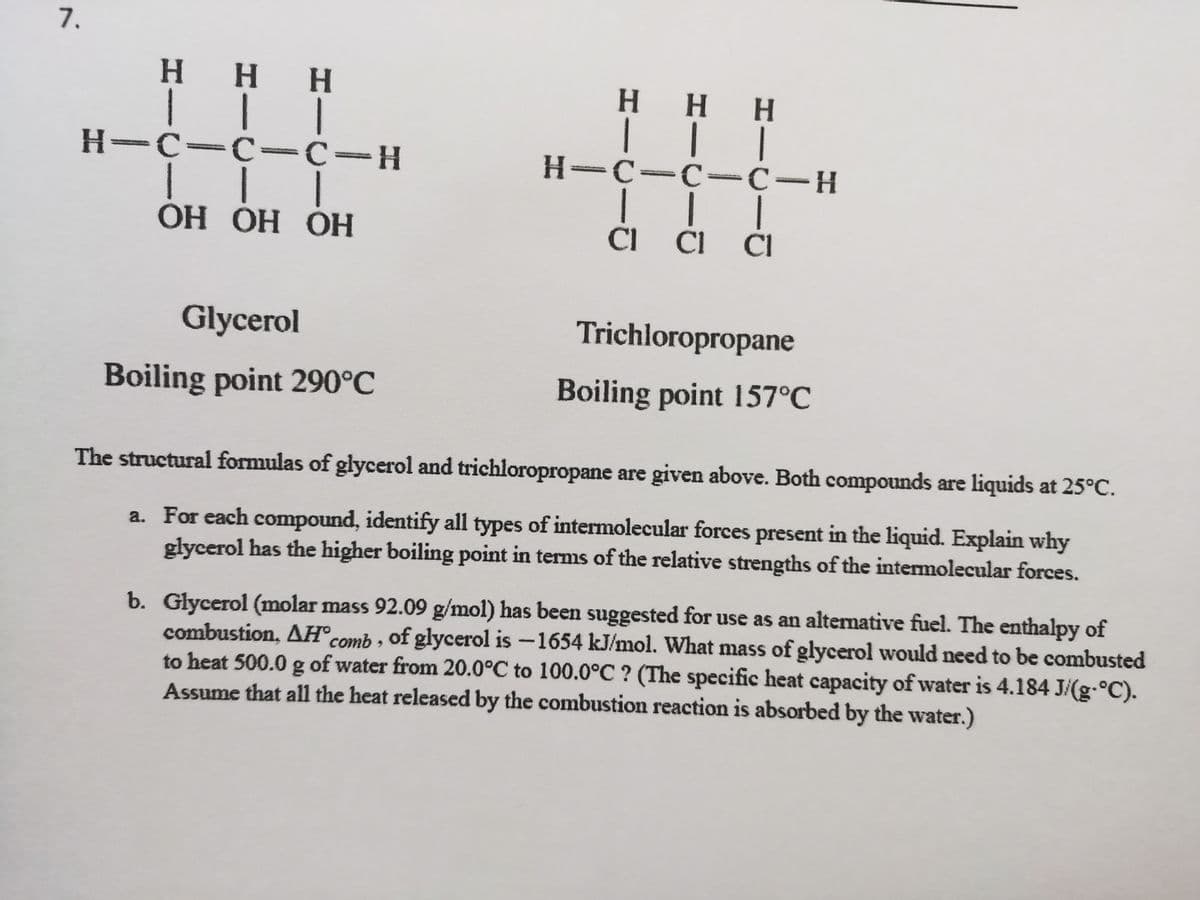
Transcribed Image Text:7.
H HH
H H H
|| |
H-C-C -C-H
H-C-C =c-H
ОН ОН ОН
CI CI CI
Glycerol
Trichloropropane
Boiling point 290°C
Boiling point 157°C
The structural formulas of glycerol and trichloropropane are given above. Both compounds are liquids at 25°C.
a. For each compound, identify all types of intermolecular forces present in the liquid. Explain why
glycerol has the higher boiling point in terms of the relative strengths of the intermolecular forces.
b. Glycerol (molar mass 92.09 g/mol) has been suggested for use as an alternative fuel. The enthalpy of
combustion, AH® comb , of glycerol is -1654 kJ/mol. What mass of glycerol would need to be combusted
to heat 500.0 g of water from 20.0°C to 100.0°C ? (The specific heat capacity of water is 4.184 J/(g-°C).
Assume that all the heat released by the combustion reaction is absorbed by the water.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning