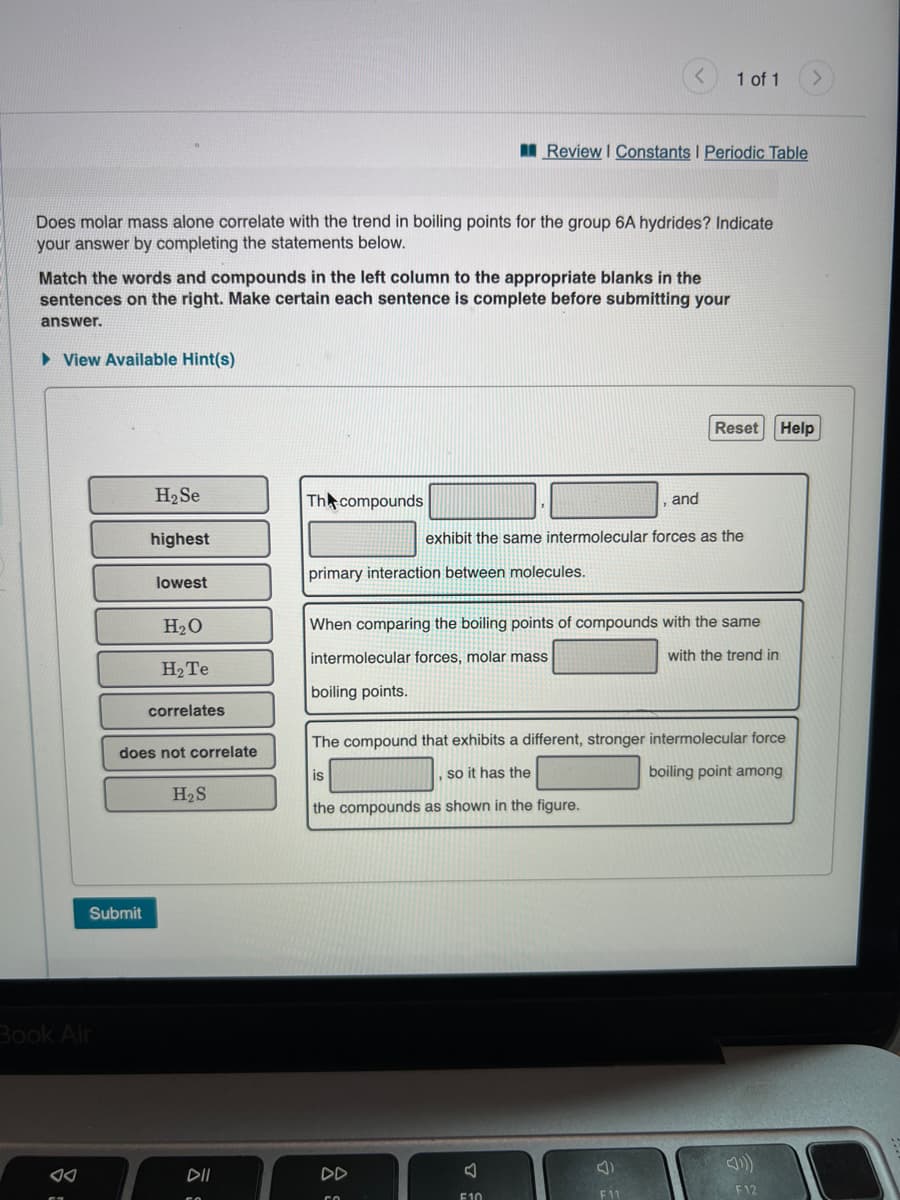
H2 Se Th compounds and highest exhibit the same intermolecular forces as the primary interaction between molecules. lowest H20 When comparing the boiling points of compounds with the same intermolecular forces, molar mass with the trend in H2TE boiling points. correlates The compound that exhibits a different, stronger intermolecular force does not correlate is so it has the boiling point among H2S the compounds as shown in the figure.
H2 Se Th compounds and highest exhibit the same intermolecular forces as the primary interaction between molecules. lowest H20 When comparing the boiling points of compounds with the same intermolecular forces, molar mass with the trend in H2TE boiling points. correlates The compound that exhibits a different, stronger intermolecular force does not correlate is so it has the boiling point among H2S the compounds as shown in the figure.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter20: The Representative Elements
Section: Chapter Questions
Problem 110CP
Related questions
Question
Need help
Transcribed Image Text:1 of 1
I Review I Constants I Periodic Table
Does molar mass alone correlate with the trend in boiling points for the group 6A hydrides? Indicate
your answer by completing the statements below.
Match the words and compounds in the left column to the appropriate blanks in the
sentences on the right. Make certain each sentence is complete before submitting your
answer.
• View Available Hint(s)
Reset Help
H2 Se
Th compounds
and
highest
exhibit the same intermolecular forces as the
primary interaction between molecules.
lowest
H20
When comparing the boiling points of compounds with the same
intermolecular forces, molar mass
with the trend in
H2 Te
boiling points.
correlates
The compound that exhibits a different, stronger intermolecular force
does not correlate
is
so it has the
boiling point among
H2S
the compounds as shown in the figure.
Submit
Book Air
DD
DII
DD
F12
E10
F11
Transcribed Image Text:e Home
Item 1
us
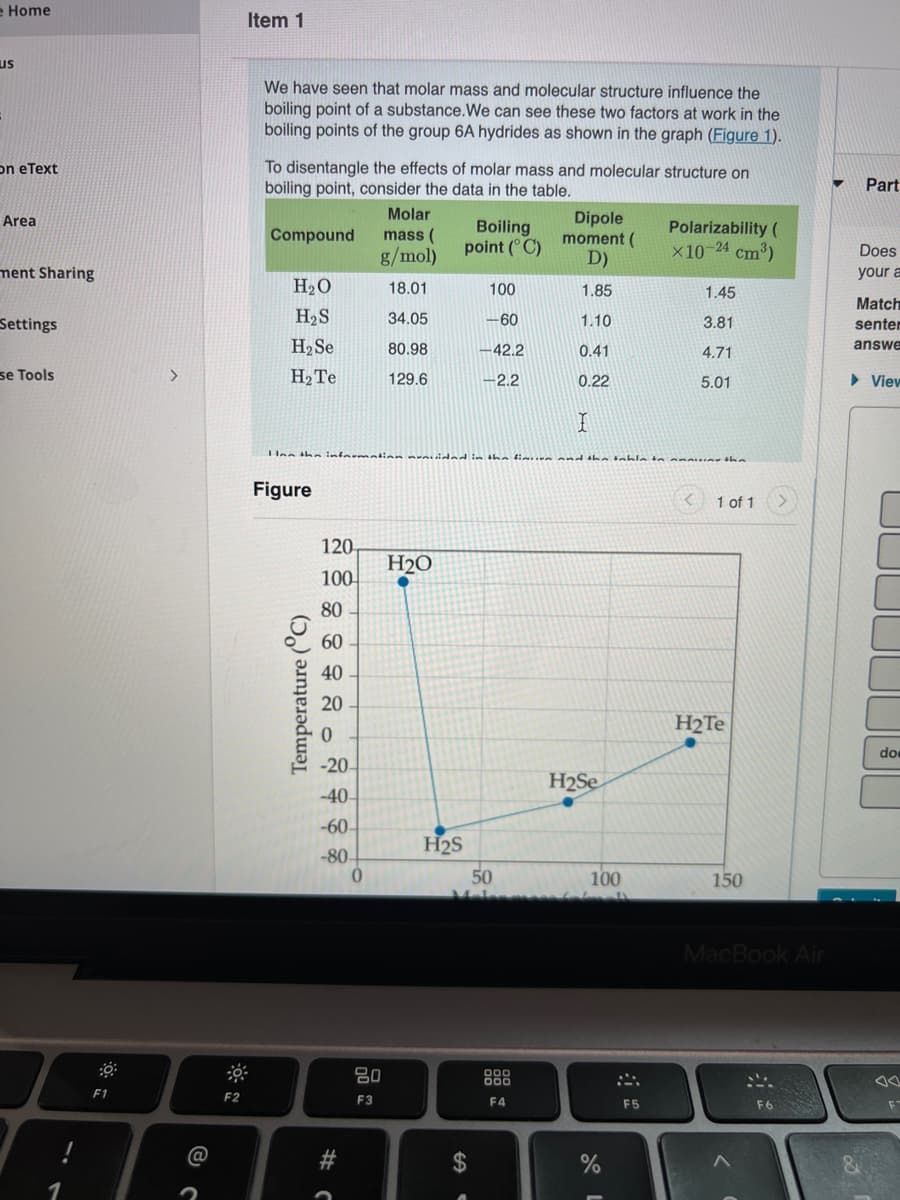
We have seen that molar mass and molecular structure influence the
boiling point of a substance.We can see these two factors at work in the
boiling points of the group 6A hydrides as shown in the graph (Figure 1).
on eText
To disentangle the effects of molar mass and molecular structure on
boiling point, consider the data in the table.
Part
Molar
Dipole
moment (
D)
Area
Boiling
point (°C)
Polarizability (
×10-24 cm3)
Compound
mass (
g/mol)
Does
ment Sharing
your a
H20
18.01
100
1.85
1.45
Match
Settings
H2S
34.05
–60
1.10
3.81
senter
H2 Se
80.98
-42.2
0.41
4.71
answe
se Tools
>
H2TE
129.6
-2.2
0.22
5.01
> View
Ilan the informatian rovidnd in the 4i n and tha tabla ta ann
Figure
< 1 of 1
120
H2O
100
80
60
40
20
H2TE
do
-20
H2Se
-40-
-60
H2S
50
-80-
100
150
MacBook Air
80
888
F1
F2
F3
F4
F5
@
%23
24
1
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning