Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.2: Cis–trans Isomerism In Cycloalkanes
Problem 5P: Draw the structures of the following molecules: (a) trans-l-Bromo-3-methylcyclohexane (b) cis-1,...
Related questions
Question
Esterication is the reaction of a carboxylic acid (RCOOH) with an alcohol (R'OH) to form an ester (RCOOR') with loss of water. Equation [1] is an example of an intermolecular esterication reaction. Equation [2] is an example of an intramolecular esterication reaction; that is, the carboxylic acid and alcohol are contained in the same starting material, forming a cyclic ester as product. The equilibrium constants for both reactions are given. Explain why Keq is different for these two apparently similar reactions.
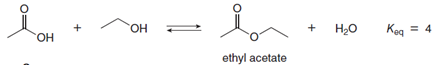
Transcribed Image Text:H20
Keg = 4
Он
ОН
ethyl acetate
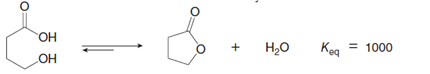
Transcribed Image Text:Kea
Н.о
1000
= 1000
ОН
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

